Introduction
Colorectal cancer (CRC) is one of the most common
and life-threatening types of cancer worldwide (1). Through the development of
multidisciplinary care and advances in surgical procedures and
techniques over the past decade (2), additional treatment strategies and
chemotherapeutic regimens have been found to be beneficial even for
advanced-stage cancer. Improved combinations of 5-fluorouracil
(5-FU)/folinic acid with irinotecan (FOLFIRI) or oxaliplatin
(FOLFOX) have progressively increased tumor response up to 73%,
while the median survival time (MST) of patients with unresectable
tumor has increased to 20 months (3).
Over the past several years, the selection of
chemotherapeutic regimens has expanded greatly and satisfactorily
due to the development of molecular-targeted therapy (4). In this context, 5-FU has become
crucial in chemotherapeutic regimens for gastrointestinal cancer
(5). 5-FU metabolic enzymes,
orotate phosphoribosyltransferase (OPRT), thymidylate synthase (TS)
and dihydropyrimidine dehydrogenase (DPD) are considered important
predictive factors of the chemotherapeutic effect even in recently
developed regimens (6–8). In the present study, the expression
of these 5-FU metabolic enzymes and their correlation with
beneficial outcomes, such as patient prognosis, as well as
unfavorable outcomes, such as potential side-effects, were
evaluated.
Materials and methods
Patients
Surgically obtained specimens from 106 CRC patients
who were operated on between January, 2004 and April, 2008 in the
Department of Surgical Oncology (Gifu University School of
Medicine, Gifu, Japan), were investigated in this study (Table I). Patients comprised 54 males and
52 females with an age range of 27–95 (mean, 64.5±14.2) years. The
disease stage was determined according to the pathological
Tumor-Node-Metastasis (TNM) staging system [International Union
Against Cancer (UICC), 7th edition]. Pathological T indicators from
Tis to T2 were observed in 20.8% of the patients. Distant
metastases including overlapping metastases at each stage were
detected at diagnosis in the lymph node, liver and lung. Peritoneal
dissemination was detected in 17.0, 32.1, 22.6 and 8.4% of
patients, respectively. As shown in Table II, 79 patients were treated with
5-FU-based chemotherapies, such as FOLFOX alone (n=22) or in
combination with bevacizumab (n=3), 5-FU with leucovorin (n=16), as
well as uracil/tegafur (UFT) alone (n=17) or in combination with
leucovorin (n=17). Side-effects of chemotherapy were classified
according to the Common Terminology Criteria for Adverse Events
(CTCAE v4.03). In this study, any event over grade 1 was regarded
as a chemo-therapy-induced side-effect. Experiments were planned
carefully in accordance with the Ethical Standards of the Helsinki
Declaration of 1975. In addition, the Institutional Research Ethics
Board of the Gifu University (Gifu, Japan) approved this
retrospective study.
 | Table IPatient clinicopathological
characteristics. |
Table I
Patient clinicopathological
characteristics.
| Characteristics | Value (n) |
|---|
| Age (years) | |
| <60 | 42 |
| ≥60 | 64 |
| Gender | |
| Male | 54 |
| Female | 52 |
| Site | |
| C-A-T | 29 |
| D-S-R | 77 |
| Differentiation | |
| Well | 57 |
| Moderate | 46 |
| Poor | 3 |
| Depth of
invasion | |
|
Mucosal-submucosal | 6 |
| Muscularis
propria-serosa infiltrating (ai) | 100 |
| Lymphatic duct
invasion | |
| 0 | 9 |
| 1 | 52 |
| 2 | 29 |
| 3 | 16 |
| Vascular
invasion | |
| 0 | 25 |
| 1 | 43 |
| 2 | 26 |
| 3 | 12 |
| All metastases | |
| (+) | 50 |
| (−) | 56 |
| Liver | |
| (+) | 34 |
| (−) | 72 |
| Lung | |
| (+) | 24 |
| (−) | 82 |
| Peritoneal
dissemination | |
| (+) | 9 |
| (−) | 97 |
| Lymph node | |
| (+) | 18 |
| (−) | 88 |
| Bone | |
| (+) | 5 |
| (−) | 101 |
| Stage (pTNM) | |
| 0–IIC | 50 |
| IIIA–IVB | 56 |
| T (pTNM) | |
| Tis-T2 | 22 |
| T3–T4b | 84 |
| N (pTNM) | |
| N0 | 50 |
| N1–2 | 56 |
| M (pTNM) | |
| M0 | 83 |
| M1 (a/b) | 23 |
 | Table IINumber of patients receiving
neoadjuvant chemotherapy. |
Table II
Number of patients receiving
neoadjuvant chemotherapy.
| Chemotherapies | Neoadjuvant
chemotherapy | No. of
patients | Total no. of
patients (n=106) |
|---|
| 5-FU-based
chemotherapies (prior to treatment with FOLFOX, FOLFIRI) | Uracil/tegafur
(UFT) alone | 17 | |
| Uracil/tegafur
(UFT) + oral leucovorin (Uzel) | 17 | |
| Uracil/tegafur
(UFT) + irinotecan (CPT-11) | 1 | |
|
Tegafur/gimeracil/oteracil potassium
(TS-1) | 1 | |
| 5-FU +
leucovorin | 16 | |
| 5′-DFUR
(Furtulon) | 2 | 54 |
| 5-FU-based
chemotherapies (following treatment with FOLFOX, FOLFIRI) | FOLFOX | 22 | |
| FOLFOX +
bevacizumab | 3 | 25 |
| Other
chemotherapies | Paclitaxel
(Taxol) | 1 | 1 |
| No
chemotherapies | None | 26 | 26 |
Immunohistochemical staining
Formalin-fixed, paraffin-embedded blocks of CRC
specimens were obtained from the Division of Clinical Pathology of
our institution, the Gifu University Graduate School of Medicine
(Gifu, Japan). Details of the immunohistochemical staining
techniques used have been previously described (9–11).
Antigen retrieval was performed by autoclaving the sections in 0.01
M citrate buffer for TS and in ethylenediaminetetraacetic acid
(EDTA) (pH 8.0) buffer for DPD for 60 sec at 120°C (DakoCytomation,
Glostrup, Denmark). Endogenous peroxidase was quenched with 0.3%
hydrogen peroxide methanol for 20 min. The immunoperoxidase
procedure [avidin-biotin-complex (ABC) method,
VECTASTAIN® Elite ABC kit; Vector Laboratories, Inc.,
Burlingame, CA, USA] was used. Primary antibodies were incubated
overnight at 4°C. 3,3′-Diaminobenzidine tetrahydrochloride (DAB)
(Dojin, Osaka, Japan) was applied for 2–3 min, and slides were
counterstained with hematoxylin. Protein expression was evaluated
by counting the number of cells with positive cytoplasmic staining
among 200 tumor cells/field in 10 fields and scored as: staining
percentage (SP) <10%, 0 points; SP≥10 and <30%, 1 point;
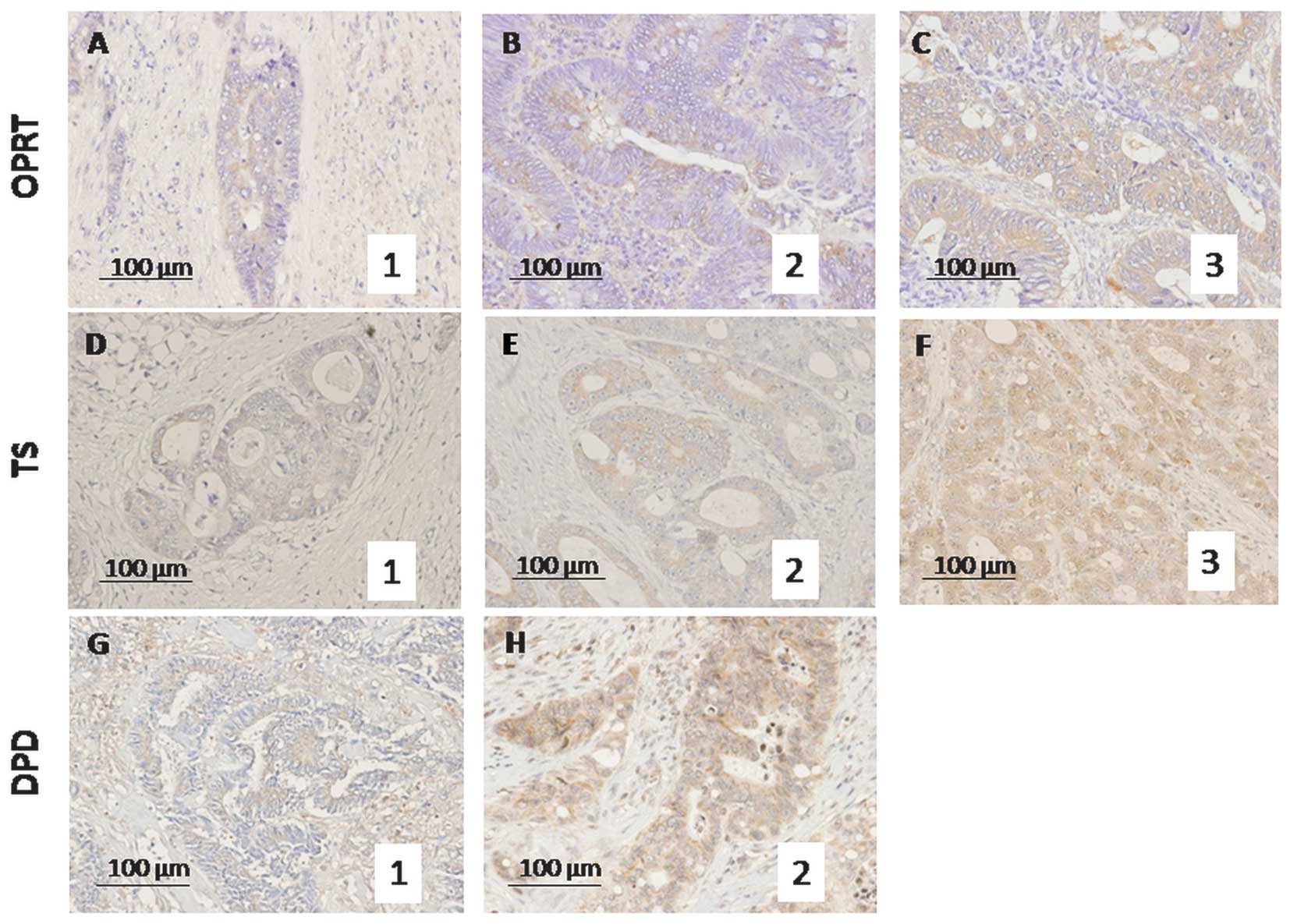
SP≥30 and 50%, 2 points and SP≥50%, 3 points (Fig. 1), as previously reported (7,12).
The average score of each category was calculated. Two co-authors
(HT and KN) blinded to any prior knowledge of the
clinicopathological parameters evaluated the stained sections, and
an expression index for each factor was calculated by averaging the
percentage of positive cytoplasmic staining (7).
Statistical analysis
Data were presented as the mean ± SD and were
evaluated for statistical significance using the Mann-Whitney U and
post-hoc tests (ANOVA). The Mann-Whitney U test was used to analyze
the correlation between two factors in the clinicopathological
data. Overall survival (OS), MST and disease-free survival (DFS)
were calculated using the Kaplan-Meier method. The Cox proportional
hazards model was used to compare these curves. P<0.05 was
considered to indicate a statistically significant difference. SPSS
version 14 (SPSS, Inc., Chicago, IL, USA) and StatView 5.0 (SAS
Institute Inc., Cary, NC, USA) were used for data management and
analysis.
Results
Correlation between clinicopathological
characteristics and protein expression grade
Positive immunohistochemical staining (SP≥10%) was
detected for OPRT in 80 (75.5%), for TS in 38 (35.8%) and for DPD
in 8 (7.5%) patients. Concerning the correlation with
clinicopathological factors (Table
III), the expression score of OPRT was lower in patients with
positive lymph node metastasis compared with patients with a
negative metastasis (0.94±1.03 vs. 1.48±1.06, P=0.0496). Expression
of DPD as detected by positive staining was markedly higher in both
the advanced TNM stage (IIIA–IVB) vs. mild stage (0–IIC) lesions
(0.14±0.40 vs. 0.02±0.14, P=0.0414) and the positive vs. negative
lymph node metastases for the N factor (0.14±0.40 vs. 0.02±0.14,
P=0.0414). No statistically significant differences were observed
in other clinicopathological characteristics, such as age, gender,
location of tumor, differentiation and depth of wall invasion.
 | Table IIICorrelation between
clinicopathological characteristics and protein expression
grade. |
Table III
Correlation between
clinicopathological characteristics and protein expression
grade.
|
Characteristics | TS | P-value | DPD | P-value | OPRT | P-value |
|---|
| Age (years) | | | | | | |
| <60 | 0.50±0.70 | | 0.07±0.34 | | 1.36±1.13 | |
| ≥60 | 0.47±0.77 | 0.5647 | 0.09±0.29 | 0.4050 | 1.41±1.03 | 0.7529 |
| Gender | | | | | | |
| Male | 0.50±0.79 | | 0.07±0.26 | | 1.44±1.07 | |
| Female | 0.46±0.69 | 0.9940 | 0.10±0.35 | 0.9340 | 1.33±1.07 | 0.5686 |
| Tumor location | | | | | | |
| C-A-T | 0.62±0.89 | | 0.07±0.25 | | 1.45±1.10 | |
| D-S-R | 0.43±0.67 | 0.3796 | 0.09±0.33 | 0.8648 | 1.36±1.06 | 0.7381 |
|
Differentiation | | | | | | |
| Well | 0.46±0.73 | | 0.09±0.34 | | 1.19±1.02 | |
| Moderate | 0.46±0.68 | | 0.09±0.28 | | 1.63±1.09 | |
| Poor | 1.33±1.25 | 0.3832 | 0.00±0.00 | 0.8462 | 1.33±0.94 | 0.1242 |
| Depth of
invasion | | | | | | |
| m-sm | 0.50±0.50 | | 0.00±0.00 | | 0.83±0.90 | |
| mp-si (ai) | 0.48±0.75 | 0.6282 | 0.09±0.32 | 0.4734 | 1.42±1.07 | 0.1995 |
| Lymphatic duct
invasion | | | | | | |
| 0 | 0.44±0.50 | | 0.00±0.00 | | 1.11±0.87 | |
| 1 | 0.48±0.84 | | 0.08±0.27 | | 1.46±1.01 | |
| 2 | 0.52±0.68 | | 0.14±0.43 | | 1.55±1.07 | |
| 3 | 0.44±0.61 | 0.6611 | 0.06±0.24 | 0.4543 | 1.00±1.22 | 0.5091 |
| Vascular
invasion | | | | | | |
| 0 | 0.36±0.69 | | 0.00±0.00 | | 1.16±0.97 | |
| 1 | 0.58±0.81 | | 0.14±0.41 | | 1.51±1.04 | |
| 2 | 0.46±0.63 | | 0.12±0.32 | | 1.50±1.15 | |
| 3 | 0.42±0.76 | 0.7229 | 0.00±0.00 | 0.3913 | 1.17±1.07 | 0.6086 |
| All metastases | | | | | | |
| (+) | 0.38±0.63 | | 0.06±0.24 | | 1.18±1.09 | |
| (−) | 0.57±0.82 | 0.2319 | 0.11±0.36 | 0.5568 | 1.57±1.02 | 0.0545 |
| Liver | | | | | | |
| (+) | 0.44±0.69 | | 0.09±0.28 | | 1.15±1.09 | |
| (−) | 0.50±0.76 | 0.7013 | 0.08±0.32 | 0.7505 | 1.50±1.04 | 0.1065 |
| Lung | | | | | | |
| (+) | 0.33±0.47 | | 0.04±0.20 | | 1.21±1.08 | |
| (−) | 0.52±0.80 | 0.5210 | 0.10±0.34 | 0.4731 | 1.44±1.06 | 0.3639 |
| Peritoneal
dissemination | | | | | | |
| (+) | 0.44±0.68 | | 0.11±0.31 | | 1.44±1.34 | |
| (−) | 0.48±0.75 | 0.8988 | 0.08±0.31 | 0.6828 | 1.38±1.04 | 0.9531 |
| Lymph node | | | | | | |
| (+) | 0.33±0.58 | | 0.00±0.00 | | 0.94±1.03 | |
| (−) | 0.51±0.77 | 0.4012 | 0.10±0.34 | 0.1856 | 1.48±1.06 | 0.0496 |
| Bone | | | | | | |
| (+) | 0.00±0.00 | | 0.20±0.40 | | 1.40±0.49 | |
| (−) | 0.50±0.75 | 0.0946 | 0.08±0.30 | 0.2900 | 1.39±1.09 | 0.8711 |
| Stage (pTNM) | | | | | | |
| 0–IIC | 0.38±0.56 | | 0.02±0.14 | | 1.38±0.98 | |
| IIIA–IVB | 0.57±0.86 | 0.4594 | 0.14±0.40 | 0.0414 | 1.39±1.14 | 0.9529 |
| T (pTNM) | | | | | | |
| Tis-T2 | 0.50±0.58 | | 0.05±0.21 | | 1.32±1.02 | |
| T3–T4b | 0.48±0.78 | 0.4646 | 0.10±0.33 | 0.5457 | 1.40±1.08 | 0.7497 |
| N (pTNM) | | | | | | |
| N0 | 0.38±0.56 | | 0.02±0.14 | | 1.38±0.98 | |
| N1–2 | 0.57±0.86 | 0.4594 | 0.14±0.40 | 0.0414 | 1.39±1.14 | 0.9529 |
| M (pTNM) | | | | | | |
| M0 | 0.47±0.75 | | 0.08±0.32 | | 1.45±1.08 | |
| M1 (a/b) | 0.52±0.71 | 0.6673 | 0.09±0.28 | 0.8277 | 1.17±1.01 | 0.3109 |
Correlation between OPRT expression and
MST, and time to recurrence
Expression of the three 5-FU metabolic enzymes was
also evaluated from the viewpoint of patient prognosis (Fig. 2). In all the cases, the OS rate was
higher in patients with a positive OPRT expression (75.5%) vs. a
negative OPRT expression (24.5%) (MST, not reached vs. 42.3 months,
P=0.0144). In the 79 patients receiving 5-FU-based chemotherapy,
the OS rate was also higher in patients with a positive OPRT
expression (75.9%) vs. a negative OPRT expression (24.1%) (MST, not
reached vs. 39.5 months, P=0.0167). Furthermore, in these 79
patients, OPRT expression was markedly higher in the patients with
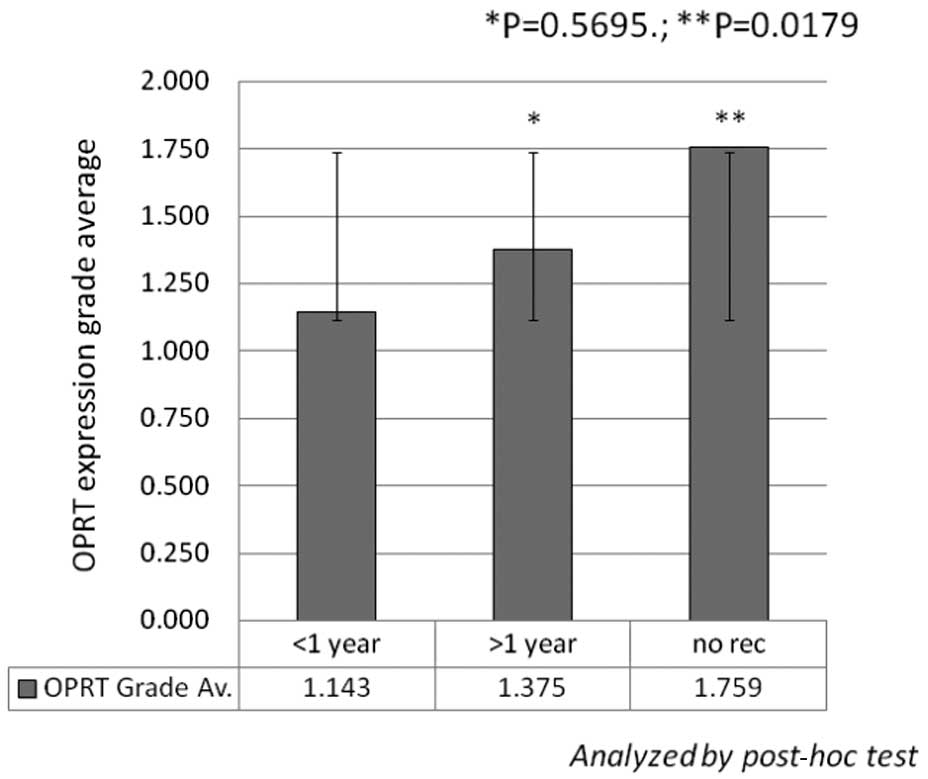
no recurrence vs. those with early recurrence (recurrence within 1
year with any metastasis at surgery) (1.759±0.951 vs. 1.143±1.049,
P=0.0179) (Fig. 3). No
statistically significant differences were detected in the
expression of DPD and TS (data not shown).
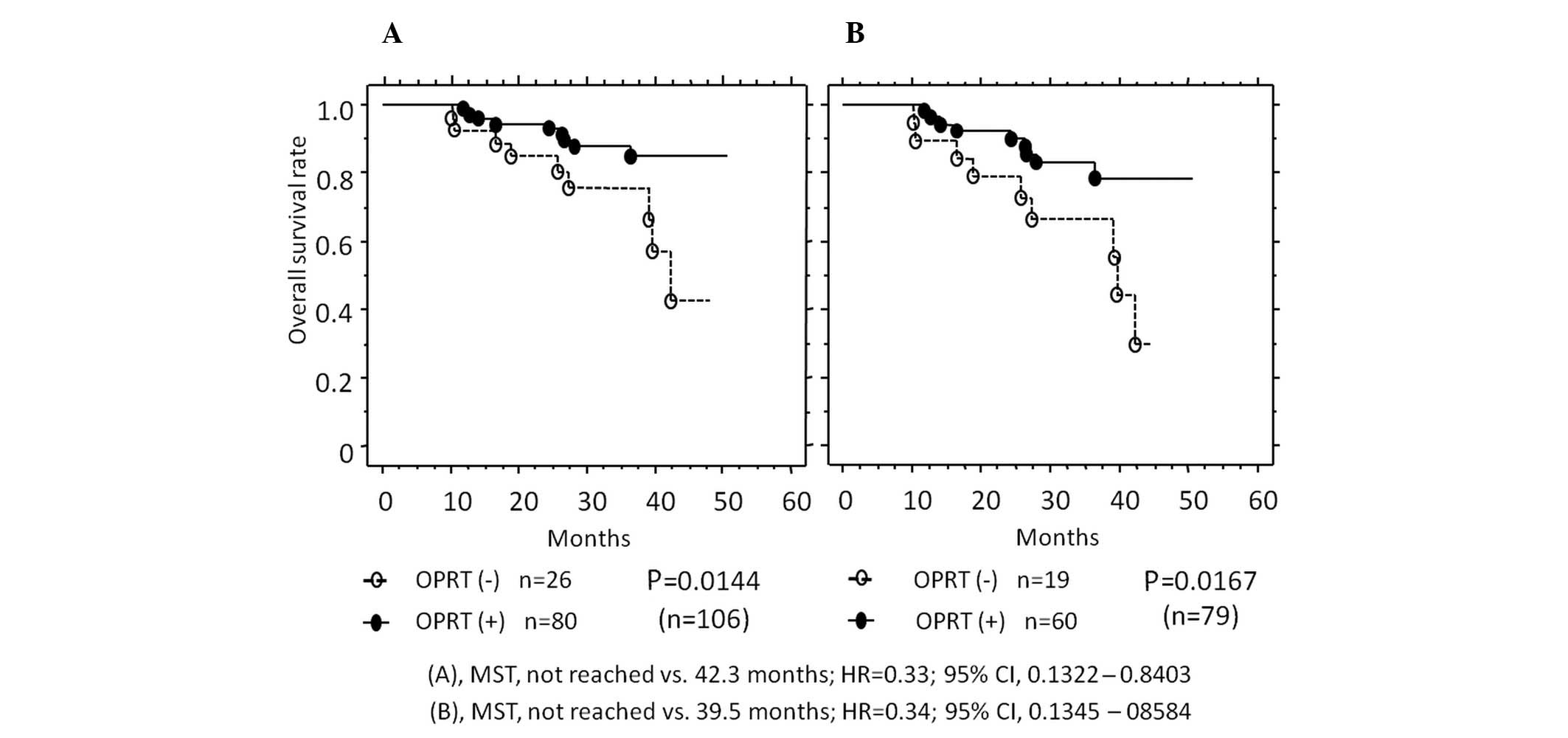 | Figure 2Kaplan-Meier curves showing the
correlation between OPRT expression and median survival time (MST).
(A) In the 106 patients, survival was improved in patients with a
positive OPRT expression (closed circles, n=80) compared with
patients with a negative expression (open circles, n=26). (B) In 79
patients treated with 5-FU-based chemotherapy, survival was
improved in patients with a positive OPRT expression (closed
circles, n=60) compared with patients with a negative expression
(open circles, n=19). OPRT, orotate phosphoribosyltransferase; HR,
hazard ratio; CI, confidence interval; 5-FU, 5-fluorouracil.. |
Correlations between enzyme expression
and side-effects, and between patient prognosis and
side-effects
Side-effects were detected in 43/79 (54.4%) patients
receiving 5-FU-based chemotherapy. Of the 49 patients undergoing R0
surgery, side-effects occurred in 20 (40.8%). The expression level
of OPRT was markedly lower in patients positive for side-effects
compared with patients negative for side-effects (1.05±0.86 vs.
1.86±1.11, P=0.0126) (Table IV).
The correlation between side-effects and patient prognosis was
compared in patients with no preoperative metastasis (n=49). DFS
was markedly better in patients without side-effects (59.2%, not
reached) compared with those with side-effects (40.8%, 13.6 months)
(P=0.0021) (Fig. 4A). Furthermore,
in patients with positive OPRT expression and with no side-effects,
DFS (not reached) was markedly improved compared with patients (not
reached vs. 13.2 months, P= 0.0031; not reached vs. 8.2 months, P=
0.0001) (Fig. 4B). No
statistically significant differences were detected in TS or DPD
expression with regard to prognosis (data not shown).
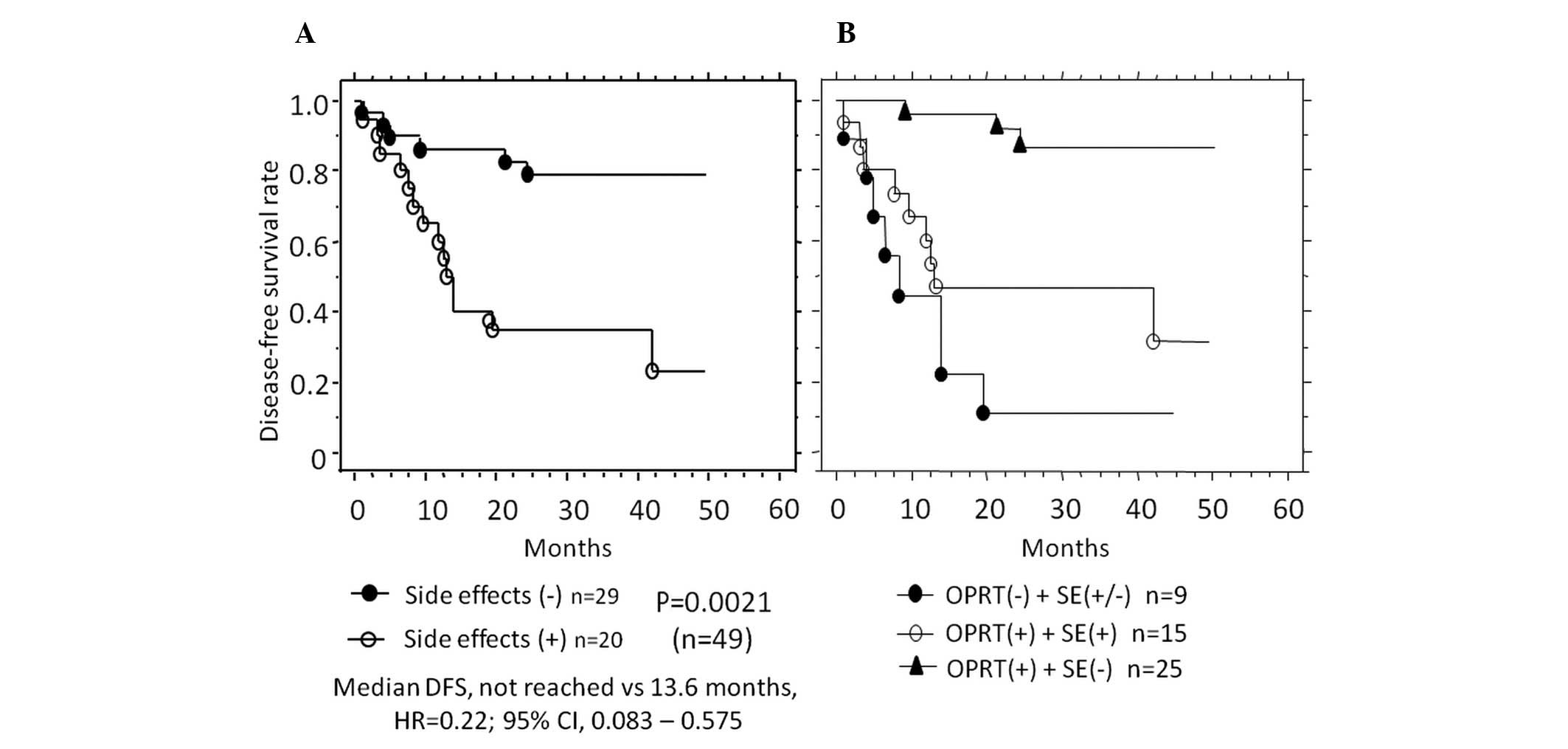 | Figure 4Kaplan-Meier curves showing patient
prognosis with regard to side-effects. (A) Disease-free survival
(DFS) after R0 surgery was improved in patients without (closed
circles, n=29) compared with patients with side-effects (open
circles, n=20). (B) DFS was improved in patients with a positive
OPRT expression and no side-effects (triangles, n=25) compared with
patients either with a negative (closed circles, n=9) or positive
expression of OPRT, and with side-effects (open circle, n=15).
OPRT, orotate phosphoribosyltransferase; SE, side-effects; HR,
hazard ratio; CI, confidence interval. |
 | Table IVCorrelation between enzyme expression
and side-effects. |
Table IV
Correlation between enzyme expression
and side-effects.
| Enzymes | Patients receiving
5-FU-based chemotherapy (n=79) | Patients with no
metastasis prior to surgery (receiving 5-FU-based chemotherapy)
(n=49) |
|---|
|
|
|---|
| Side-effects (+)
(n=43) | Side-effects (−)
(n=36) | P-value | Side-effects (+)
(n=20) | Side-effects (−)
(n=29) | P-value |
|---|
| OPRT | 1.19±0.92 | 1.64±1.18 | 0.0908 | 1.05±0.86 | 1.86±1.11 | 0.0126 |
| TS | 0.44±0.69 | 0.53±0.87 | 0.925 | 0.45±0.74 | 0.52±0.86 | 0.9323 |
| DPD | 0.04±0.21 | 0.17±0.44 | 0.1473 | 0 | 0.17±0.46 | 0.0865 |
Discussion
Combination chemotherapies with 5-FU/folinic acid
have progressively improved tumor response and MST. According to
the National Cancer Comprehensive Network (NCCN)
Guidelines® (Colon cancer, version 3, 2009; Rectal
cancer, version 2, 2012), 5-FU is a key chemotherapeutic agent for
CRC. However, the importance of 5-FU-related metabolic enzymes in
the evaluation of patient outcome with respect to CRC patient
prognosis remains controversial. In the present study, due to the
fact that adjuvant chemotherapy for stage IIB/C CRC, considered a
high-risk group for recurrence (13), is recommended but not required,
patients were assessed according to TNM classification as IIIA–IVB
patients with and 0–IIC patients without chemotherapy. Cancer
progression was observed to have a significant correlation with
both OPRT and DPD expression, although not with TS expression.
Furthermore, OPRT expression, but not TS and DPD expression, was
identified as a predictor of patient prognosis and occurrence of
side-effects caused by 5-FU-based chemotherapy. Of these three
metabolic factors, the expression of OPRT protein was more easily
detected in primary CRC with progressive depth of invasion or lymph
node metastasis in gastrointestinal cancer (12,14,15),
even in the early stages of carcinogenesis (16,17).
Therefore, OPRT might be valuable as a predictive indicator of
chemotherapeutic effects. The significance of TS or DPD expression
has been controversial over the past decade (18,19),
and a recent study estimated TS to be critical for patient
prognosis in pancreatic and breast cancers (7,20).
Furthermore, although DPD expression was associated with the
progression of carcinogenesis in lymphatic/venous invasion in
esophageal cancer or CRC (14,17),
DPD itself has yet to be identified as a predictor of the effect of
5-FU-based chemotherapy.
OPRT is known to be one of the metabolic enzymes
necessary for the conversion of 5-FU to its active nucleotide type,
and several previous studies evaluated OPRT expression in mRNA
(6,21). The present study evaluated the
expression of OPRT and has shown a correlation between OPRT protein
expression and patient prognosis. Our results were based on the
response to 5-FU as a cell-killing agent, and with regard to
side-effects. When severe side-effects occur, chemotherapy should
be discontinued, despite its effectiveness in inhibiting cancer
growth. Previous studies of OPRT mRNA expression have demonstrated
a correlation between the expression of this metabolic enzyme and
the occurrence of side-effects in patients treated with 5-FU-based
chemotherapy (22–24). According to an experimental study
evaluating these factors (25),
protein expression was more important as compared with mRNA
expression in demonstrating enzyme activity. Thus, the present
study suggests OPRT protein expression to be a predictor of the
occurrence of side-effects.
Both 2-fluoro-β-alanine (F-β-alanine) and
5-fluoro-deoxyuridine diphosphate (FdUMP), metabolized by DPD and
OPRT, respectively, were reported to induce neurological and
gastrointestinal toxicity, as well as hematotoxicity (26–28).
It is reasonable to expect that a decrease in the dose of
F-β-alanine, which induces neurological toxicity, results in a
reduction of side-effects. However, FdUMP has two opposing effects:
first, as an anticancer agent, and second, as a factor in
gastrointestinal toxicity and hematotoxicity, although an
anticancer drug with few side-effects as well as increased
carcinostatic effects is ideal for cancer patients. Consequently,
future studies verifying the mechanism of FdUMP and its
interactions with other factors in humans are required.
In conclusion, OPRT expression was found to be a
predictive factor of patient prognosis and occurrence of
side-effects in CRC patients administered 5-FU-based chemotherapy.
Following the detailed investigation of specimens resected
surgically or by endoscopic biopsy, clinicians may be able to
determine the most effective chemotherapeutic regimen.
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
DFS
|
disease-free survival
|
|
DPD
|
dihydropyrimidine dehydrogenase
|
|
MST
|
median survival time
|
|
OPRT
|
orotate phosphoribosyltransferase
|
|
OS
|
overall survival
|
|
SP
|
staining percentage
|
|
TS
|
thymidylate synthase
|
Acknowledgements
The authors would like to thank the
Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan) for kindly providing
the OPRT, TS and DPD antibodies.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 62:10–29. 2012.
|
|
2.
|
Osada S, Imai H, Sasaki Y, et al: Strategy
for synchronous and multiple liver metastasis.
Hepatogastroenterology. 59:198–203. 2012.PubMed/NCBI
|
|
3.
|
de Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.
|
|
4.
|
Giantonio BJ, Catalano PJ, Meropol NJ, et
al: Bevacizumab in combination with oxaliplatin, fluorouracil, and
leucovorin (FOLFOX4) for previously treated metastatic colorectal
cancer: results from the Eastern Cooperative Oncology Group Study
E3200. J Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar
|
|
5.
|
Hamilton SR: Targeted therapy of cancer:
new roles for pathologists in colorectal cancer. Mod Pathol.
21:S23–S30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yamada H, Iinuma H and Watanabe T:
Prognostic value of 5-fluorouracil metabolic enzyme genes in Dukes’
stage B and C colorectal cancer patients treated with oral
5-fluorouracil-based adjuvant chemotherapy. Oncol Rep. 19:729–735.
2008.PubMed/NCBI
|
|
7.
|
Komori S, Osada S, Mori R, et al:
Contribution of thymidylate synthase to gemcitabine therapy for
advanced pancreatic cancer. Pancreas. 39:1284–1292. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Komori S, Osada S and Yoshida K: Novel
strategy with gemcitabine for advanced pancreatic cancer. ISRN
Oncol. 2011:9368932011.PubMed/NCBI
|
|
9.
|
Tomita H, Yamada Y, Oyama T, et al:
Development of gastric tumors in Apc(Min/+) mice by the activation
of the beta-catenin/Tcf signaling pathway. Cancer Res.
67:4079–4087. 2007.
|
|
10.
|
Osada S, Saji S and Kuno T: Clinical
significance of combination study of apoptotic factors and
proliferating cell nuclear antigen in estimating the prognosis of
hepatocellular carcinoma. J Surg Oncol. 85:48–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Osada S, Saji S and Takahashi T: A case
report of papilla Vater carcinoma showing positive expression of
thymidine phosphorylase. Hepatogastroenterology. 51:375–377.
2004.PubMed/NCBI
|
|
12.
|
Kawahara A, Akagi Y, Hattori S, et al:
Higher expression of deoxyuridine triphosphatase (dUTPase) may
predict the metastasis potential of colorectal cancer. J Clin
Pathol. 62:364–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Van Cutsem EJ and Oliveira J: Colon
cancer: ESMO clinical recommendations for diagnosis, adjuvant
treatment and follow-up. Ann Oncol. 19:ii29–ii30. 2008.
|
|
14.
|
Tokunaga Y, Sasaki H and Saito T: Clinical
role of orotate phosphoribosyl transferase and dihydropyrimidine
dehydrogenase in colorectal cancer treated with postoperative
fluoropyrimidine. Surgery. 141:346–353. 2007. View Article : Google Scholar
|
|
15.
|
Ochiai T, Sugitani M, Nishimura K, et al:
Impact of orotate phosphoribosyl transferase activity as a
predictor of lymph node metastasis in gastric cancer. Oncol Rep.
14:987–992. 2005.PubMed/NCBI
|
|
16.
|
Sanada Y, Yoshida K, Hihara J and Okada M:
Expression of orotate phosphoribosyltransferase in colorectal
carcinoma: An immunohistochemical analysis in several components of
neoplastic lesions. Oncol Rep. 20:1005–1011. 2008.
|
|
17.
|
Tsutani Y, Yoshida K, Sanada Y, et al:
Dihydropyrimidine dehydrogenase and orotate
phosphoribosyltransferase in esophageal cancer patients:
Correlation with clinicopathological factors and prognosis. Mol Med
Rep. 1:713–719. 2008.
|
|
18.
|
Ichikawa W, Uetake H, Shirota Y, et al:
Combination of dihydropyrimidine dehydrogenase and thymidylate
synthase gene expressions in primary tumors as predictive
parameters for the efficacy of fluoropyrimidine-based chemotherapy
for metastatic colorectal cancer. Clin Cancer Res. 9:786–791.
2003.
|
|
19.
|
Ichikawa W, Uetake H, Shirota Y, et al:
Both gene expression for orotate phosphoribosyltransferase and its
ratio to dihydropyrimidine dehydrogenase influence outcome
following fluoropyrimidine-based chemotherapy for metastatic
colorectal cancer. Br J Cancer. 89:1486–1492. 2003. View Article : Google Scholar
|
|
20.
|
Hosono Y, Osada S, Nawa M, et al:
Combination therapy of 5-fluorouracil with rapamycin for hormone
receptor-negative human breast cancer. Anticancer Res.
30:2625–2630. 2010.PubMed/NCBI
|
|
21.
|
Ochiai T, Nishimura K, Noguchi H, et al:
Prognostic impact of orotate phosphoribosyl transferase among
5-fluorouracil metabolic enzymes in resectable colorectal cancers
treated by oral 5-fluorouracil-based adjuvant chemotherapy. Int J
Cancer. 118:3084–3088. 2006. View Article : Google Scholar
|
|
22.
|
Lecomte T, Ferraz JM, Zinzindohoue F, et
al: Thymidylate synthase gene polymorphism predicts toxicity in
colorectal cancer patients receiving 5-fluorouracil-based
chemotherapy. Clin Cancer Res. 10:5880–5888. 2004. View Article : Google Scholar
|
|
23.
|
Ichikawa W, Takahashi T, Suto K, Sasaki Y
and Hirayama R: Orotate phosphoribosyltransferase gene polymorphism
predicts toxicity in patients treated with bolus 5-fluorouracil
regimen. Clin Cancer Res. 12:3928–3934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
van Kuilenburg AB: Dihydropyrimidine
dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur
J Cancer. 40:939–950. 2004.PubMed/NCBI
|
|
25.
|
Tsuchida M, Yamato Y, Hashimoto T, et al:
Expression of 5-fluorouracil-related enzymes in lung cancer: ELISA
characterizes enzyme activity and messenger RNA expression. Oncol
Rep. 21:1037–1043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Arellano M, Malet-Martino M, Martino R and
Spector T: 5-Ethynyluracil (GW776): effects on the formation of the
toxic catabolites of 5-fluorouracil, fluoroacetate and
fluorohydroxypropionic acid in the isolated perfused rat liver
model. Br J Cancer. 76:1170–1180. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Yoshisue K, Hironaga K, Yamaguchi S,
Yamamoto A, Nagayama S and Kawaguchi Y: Reduction of 5-fluorouracil
(5-FU) gastrointestinal (GI) toxicity resulting from the protection
of thymidylate synthase (TS) in GI tissue by repeated simultaneous
administration of potassium oxonate (Oxo) in rats. Cancer Chemother
Pharmacol. 46:51–56. 2000. View Article : Google Scholar
|
|
28.
|
Kato T, Shimamoto Y, Uchida J, et al:
Possible regulation of 5-fluorouracil-induced neuro- and oral
toxicities by two biochemical modulators consisting of S-1, a new
oral formulation of 5-fluorouracil. Anticancer Res. 21:1705–1712.
2001.PubMed/NCBI
|