Introduction
The liver is a frequent site for the metastasis of
cancer cells originating from other sites. A significant number of
leukemic or lymphoma patients have liver metastasis (1). Notably, liver enlargement with
malignant infiltration in leukemic and lymphoma patients is
associated with poor prognosis (2). Inhibition of the interaction between
liver cells and infiltrated malignant cells may provide a novel
therapeutic method for patients diagnosed with leukemic liver
metastasis.
The CD44 transmembrane glycoprotein is ubiquitously
expressed in cells and tissues and is involved in inflammation
(3) and tumorigenesis (4). Due to alternative splicing of various
exons, CD44 is usually expressed in a variety of isoforms (CD44v)
(5). The CD44 isoforms contain a
hyaluronan (HA)-binding domain in the extracellular domain
(6). HA is a major component of
the extracellular matrix (ECM), and interacts with a variety of ECM
and cell membrane proteins (6–10),
among which link protein is a component of ECM interacting with HA
through its HA-binding motif (16). As an important cell adhesion
molecule, CD44 mediates cell-cell and cell-ECM interaction,
inducing intracellular signaling events (11,12).
Notably, the ligation of CD44 has been shown to induce the
activation or expression of intracellular signaling elements, such
as MDR1, phosphoinositide 3 kinase (PI3K) (13), Lyn (14), Ca2+ mobilization
(15) and ERK (16), which are involved in apoptosis
inhibition and chemoresistance in tumors.
In this study, an in vitro coculture system
of leukemic and liver cells was established, determined to be
mediated by the interaction of CD44 and HA. A fusion protein
containing enhanced green fluorescent protein (EGFP) and a link
protein HA-binding motif (EGFP-L) were used to detect cell membrane
HA on liver cells and block the adhesion of CD44-positive leukemia
with liver cells.
Materials and methods
Cells
The Kasumi-1 human acute myeloid leukemia cell line
was kindly provided by Professor S.J. Chen (Shanghai Jiaotong
University, Shanghai, China) and maintained in RPMI-1640 medium
(Hyclone Laboratories, Inc., Logan, UT, USA) supplemented with 20%
fetal bovine serum (FBS) (Life Technologies, Inc., Grand Island,
NY, USA) and 1% L-glutamine (Life Technologies, Inc.). The HL-60
human acute myeloid leukemia cell line was purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA) and
maintained in RPMI-1640 medium (Hyclone Laboratories, Inc.)
supplemented with 10% FBS (Life Technologies, Inc.) and 1%
L-glutamine (Life Technologies, Inc.). The L02 human fetal liver
cell line and the BEL7404 human hepatoma cell line were purchased
from the Shanghai Cell Collection (Shanghai, China) and cultured in
Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad,
CA, USA), supplemented with 10% FBS (Life Technologies, Inc.) and
1% L-glutamine (Life Technologies, Inc.).
Plasmid construction and recombinant
protein preparation
A sequence encoding EGFP was amplified from plasmid
pEGFP-C1 using PCR. The sense primer contained an NdeI site
(5′-TATCATATGGTGAGCAAGGGCGAG-3′), while the antisense primer
contained an XhoI site (5′-TATCTCGAGTT AGCATCTGAGTCC-3′).
The EGFP-L fusion gene containing a sequence encoding EGFP and a
link protein HA-binding motif with the amino acid sequence
316RYPISRPRKR325(17) was generated by PCR from pEGFP-C1.
The sense primer contained an NdeI site (5′-TATCATATGGTGAGCA
AGG GCGAG-3′), while the antisense primer contained a sequence
encoding the link protein HA-binding motif and an XhoI site
(5′-TATCTCGAGTTAGCGCTTTCTGGGTCTGGAGATGGGGTAGCGAGATCTGAGTCCGGACT-3′).
PCR products were inserted into the corresponding site of plasmid
pET-28α to generate plasmids pET-28α-EGFP and pET-28α-EGFP-L.
The plasmid pET-28α-EGFP or pET-28α-EGFP-L was
transformed into Escherichia coli strain M15 and the
expression of recombinant proteins was induced by 0.08 mM of
isopropyl-β-D-thiogalactopyranoside (IPTG) at room temperature for
6 h. Cells were harvested by centrifugation at 7,104 × g for 15 min
followed by resuspension in PBS and disruption by sonication.
Bacterial debris was pelleted by centrifugation at 15,984 × g for
15 min. Supernatants were mixed with Ni-NTA slurry (Merck
Biosciences, Darmstadt, Germany). The lysate-Ni-NTA mixture was
loaded into a column and washed twice with a washing buffer
containing 300 mM NaCl, 50 mM sodium phosphate buffer and 20 mM
imidazole (pH 8.0). The column was eluted with an elution buffer
containing 300 mM NaCl, 50 mM sodium phosphate buffer and 250 mM
imidazole (pH 8.0). The eluted protein was dialyzed against PBS at
4°C overnight to remove imidazole. Proteins (∼30 kDa) were then
analyzed by SDS-PAGE followed by Coomassie Brilliant Blue staining.
The protein concentration was determined using the BCA Protein
Assay kit (Thermo Fisher Scientific, Inc., Rockford, IL, USA).
Flow cytometry
For the detection of CD44 expression, Kasumi-1 or
HL-60 cells were incubated with fluorescein isothiocyanate
(FITC)-conjugated anti-CD44 antibody (BD Biosciences, San Jose, CA,
USA) at 4°C for 20 min. FITC-conjugated mouse IgG staining served
as the control. Following staining, the cells were analyzed on a BD
FACSAria flow cytometer (BD Biosciences).
EGFP-L staining analysis
L02 or BEL7404 cells were cultured in 24-well
plates. When cells reached 60–70% confluence, the culture medium
was aspirated and 150 μg/ml of EGFP-L was added. Following
20 min of incubation at 4°C, EGFP-L was removed. Cells were then
washed with PBS three times. EGFP staining served as the control. A
fluorescent microscope (Olympus Corporation, Tokyo, Japan) was used
to observe green fluorescence.
Cell-cell adhesion assay
L02 cells were cultured in 24-well plates. When
cells reached 60–70% confluence, the culture medium was aspirated,
and Kasumi-1 or HL-60 cells were added. After 30–45 min, any
Kasumi-1 or HL-60 cells that were non-adherent to L02 cells were
removed. A microscope (Olympus Corporation) was used to observe
cell-cell adhesion.
For the HA blocking experiments, Kasumi-1 cells were
incubated with 1 mg/ml of HA at 4°C for 10 min. Cells were then
added to the same volume of culture medium followed by coculture
with L02 precultured in 24-well plates. After 30 min, Kasumi-1
cells that were non-adherent to L02 cells were collected and
counted. PBS treatment served as the control.
For the EGFP-L blocking experiments, L02 cells
cultured in 24-well plates were incubated with 150 μg/ml of
EGFP-L or EGFP at 4°C for 30 min. Kasumi-1 cells were then added.
After 45 min, Kasumi-1 cells that were non-adherent to L02 cells
were collected and counted.
Statistical analysis
Differences among the treatment groups were assessed
by ANOVA. P<0.05 was considered to indicate a statistically
significant difference.
Results
Leukemic cells adhere to liver cells
through CD44
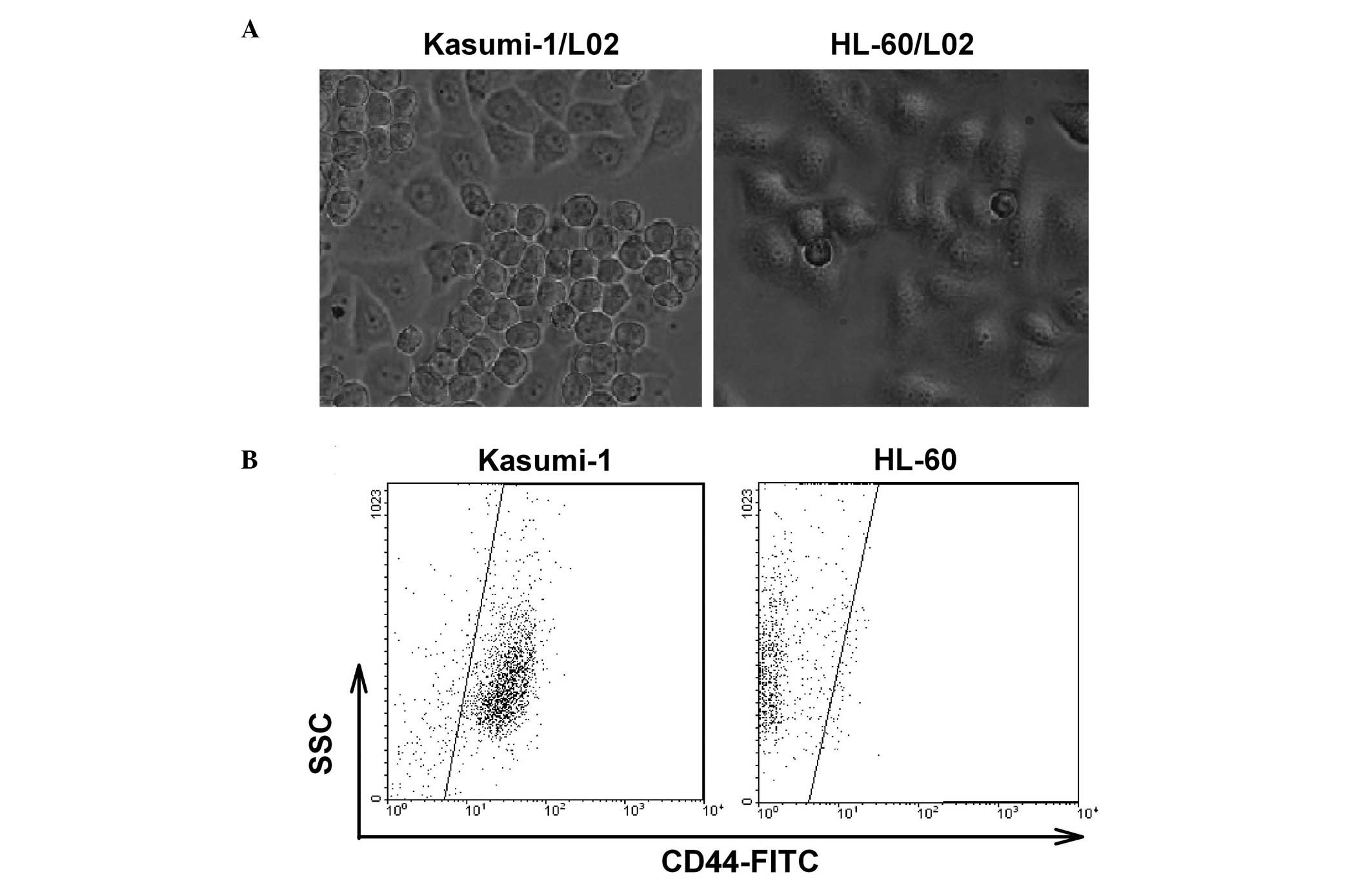
The Kasumi-1 leukemia cell line adhered to L02 liver
cells. However, this cell-cell adhesion did not occur in HL-60
cells (Fig. 1A). By analyzing the
expression of cell membrane proteins, we determined that the
majority of the Kasumi-1 cells expressed CD44, and most of the
HL-60 cells had a CD44-negative phenotype (Fig. 1B). Therefore, we hypothesized that
leukemic cells may adhere to L02 cells through CD44. To examine
this hypothesis, HA, a natural ligand for CD44 was used to pretreat
Kasumi-1 cells followed by the coculture of Kasumi-1 with L02
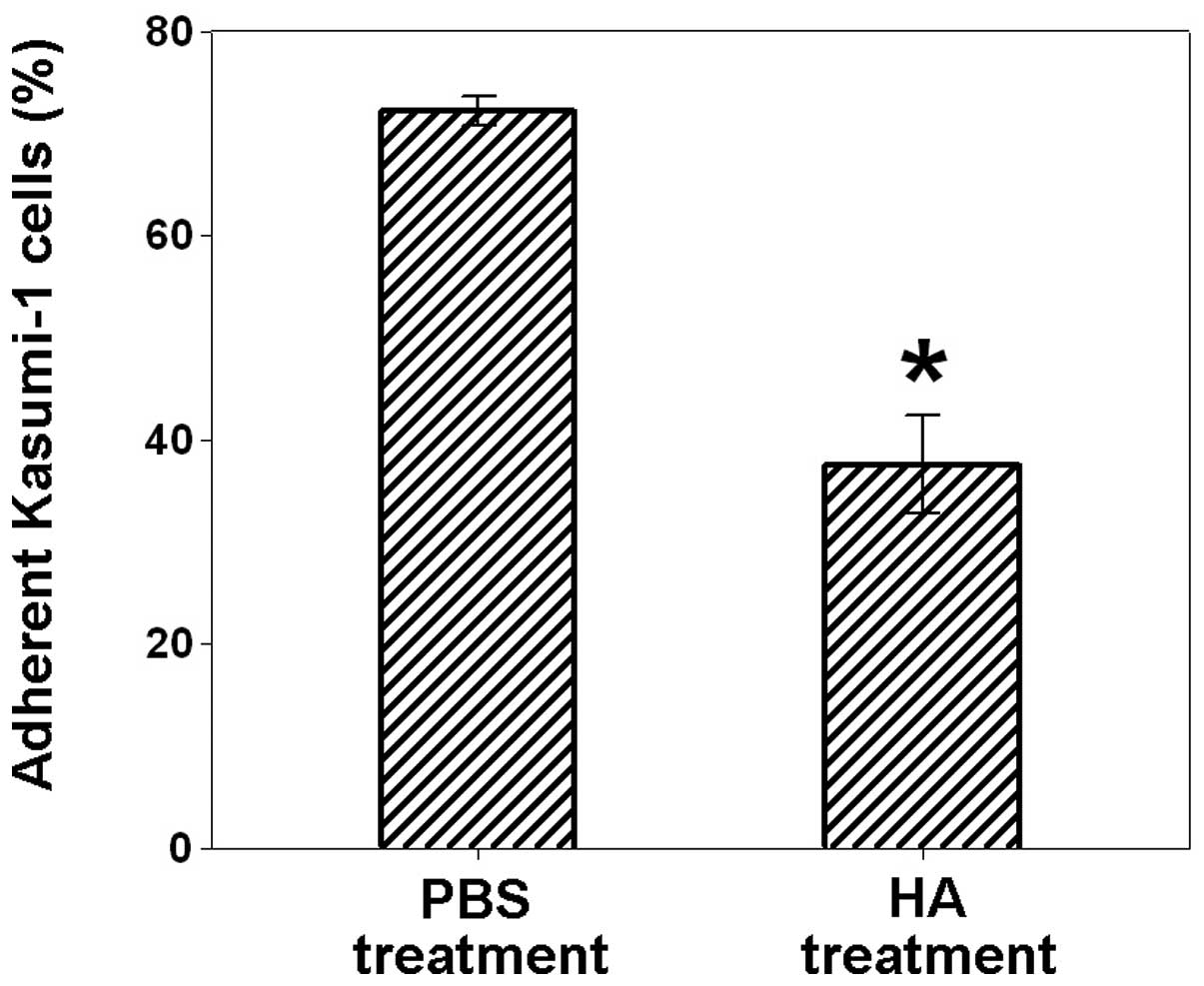
cells. As shown in Fig. 2, the
treatment of Kasumi-1 cells with HA significantly blocked the
adhesion of Kasumi-1 with L02 cells (37.6±4.8% of adherent Kasumi-1
cells), compared to the PBS control (72.3±1.4% of adherent Kasumi-1
cells). These results demonstrated that CD44 is involved in the
adhesion of leukemic cells with liver cells.
Liver cells express HA on the cell
membrane
HA is a linear glycosaminoglycan with the molecular
mass of a few million Daltons and can be secreted by cells to the
ECM or associated with the plasma membrane to form a pericellular
coat (18). Certain
cell-associated and ECM proteins, such as CD44, link protein and
collagen VI (6–10) have been found to mediate the
biological functions of HA. To determine the liver cell membrane
component involved in the adhesion with CD44-positive leukemia
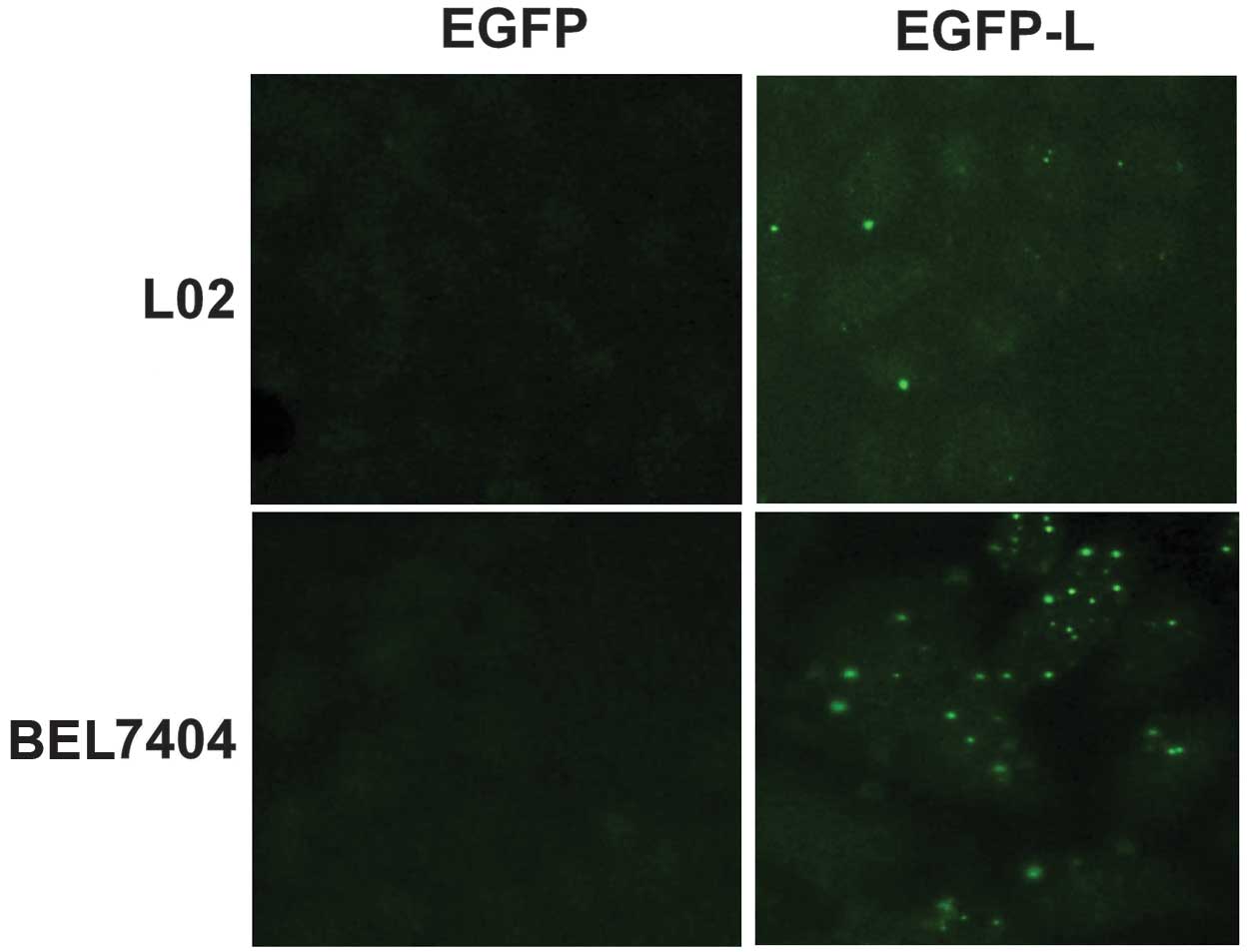
cells, EGFP-L, a fusion protein containing EGFP and a link protein
HA-binding motif were generated to detect the cell membrane HA on
liver cells. Recombinant EGFP was used as the control. The majority
of L02 and BEL7404 cells were labeled with EGFP-L as compared to
the EGFP control, indicating that liver cells L02 and BEL7404
expressed HA on the cell membrane (Fig. 3). Notably, EGFP-L formed bright
dots on the cell membrane of L02 and BEL7404 cells, suggesting that
HA may associate with certain special membrane structures in liver
cells. Taken together, these results demonstrated that the CD44-HA
interaction was involved in the adhesion of leukemic cells with
liver cells.
EGFP-L abrogates the adhesion of leukemic
and liver cells
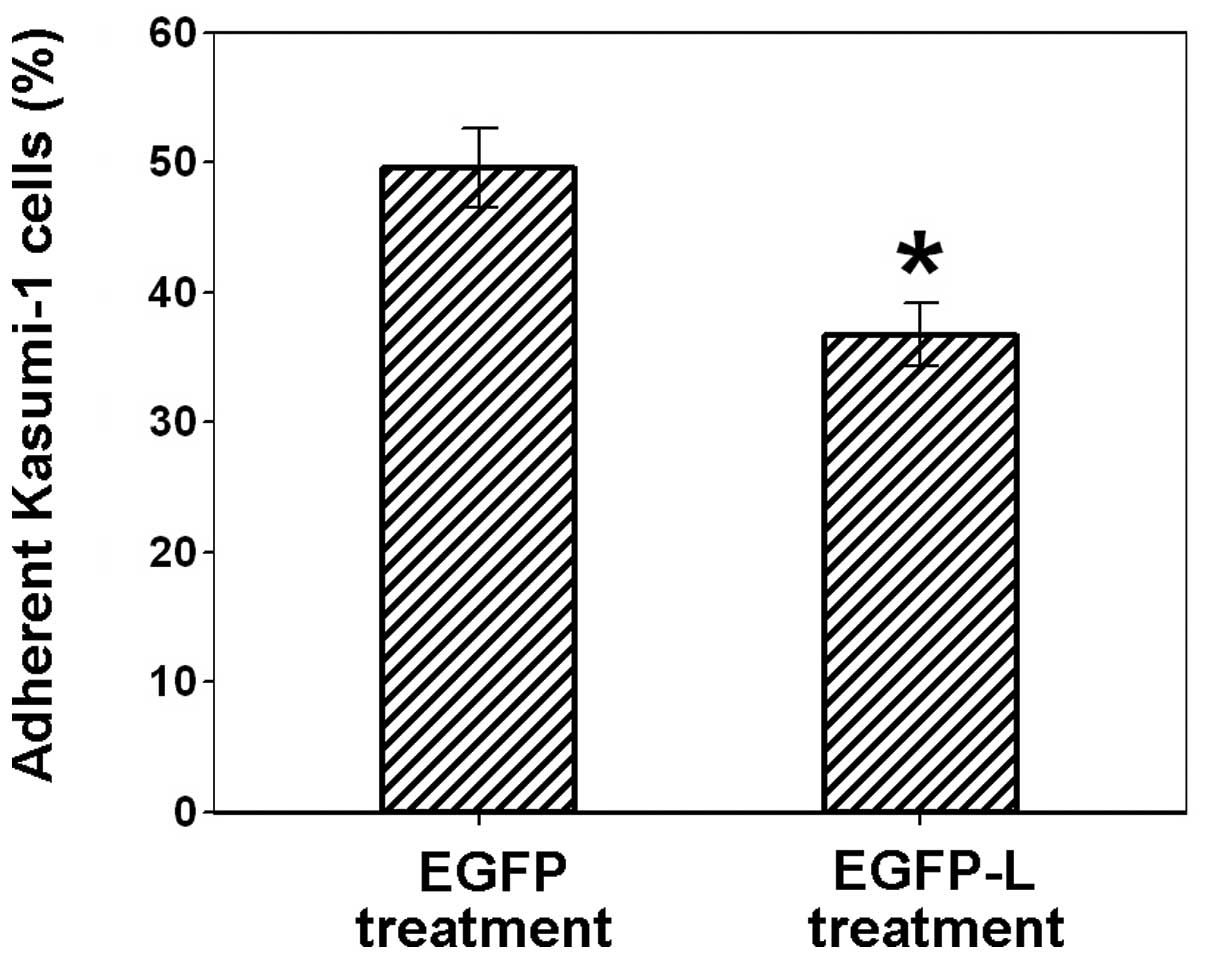
To determine the effect of EGFP-L on the adhesion of
Kasumi-1 with L02 cells, L02 cells were pretreated with EGFP-L and
then cocultured with Kasumi-1 cells. Recombinant EGFP was used as
the control. As shown in Fig. 4,
treatment of L02 cells with EGFP-L significantly blocked the
adhesion of L02 cells with Kasumi-1 cells (36.8±2.5% of adherent
Kasumi-1 cells), compared to EGFP treatment (49.6±3.0% of adherent
Kasumi-1 cells). These results indicate that the link protein
HA-binding motif is effective in inhibiting the adhesion between
CD44-positive leukemia cells with HA-positive liver cells.
Discussion
Ligation of CD44 with HA is associated with the drug
resistance of cancer cells. Furthermore, CD44-HA interaction
activates ErbB2 and PI3K to stimulate multidrug transporter MDR1,
resulting in the drug resistance of breast and ovarian cancer cells
(13,19). CD44 and MDR1 have been found to be
coexpressed in small cell lung cancer chemoresistant cells
(20). Drug resistance induced by
the CD44-HA interaction has also been observed in head and neck
cancer (15,16). Notably, the ligation of CD44 with
monoclonal antibodies has been demonstrated to decrease
drug-induced apoptosis in acute myeloid leukemia cells (21). The results of this study have shown
that the link protein HA-binding motif bound with HA in liver cell
membranes and abrogated the adhesion of CD44-positive leukemia
cells with liver cells. These findings may provide insight into
developing novel methods to inhibit liver metastasis in
leukemia.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (30801379).
References
|
1.
|
Scheimberg IB, Pollock DJ, Collins PW, et
al: Pathology of the liver in leukaemia and lymphoma. A study of
110 autopsies. Histopathology. 26:311–321. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Frei E III, Fritz RD, Price E, et al:
Renal and hepatic enlargement in acute leukemia. Cancer.
16:1089–1092. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Khan AI, Kerfoot SM, Heit B, et al: Role
of CD44 and hyaluronan in neutrophil recruitment. J Immunol.
173:7594–7601. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Naor D, Sionov RV and Ish-Shalom D: CD44:
structure, function, and association with the malignant process.
Adv Cancer Res. 71:241–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Screaton GR, Bell MV, Jackson DG, et al:
Genomic structure of DNA encoding the lymphocyte homing receptor
CD44 reveals at least 12 alternative spliced exons. Proc Natl Acad
Sci USA. 89:12160–12164. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Underhill C: CD44: the hyaluronan
receptor. J Cell Sci. 103:293–298. 1992.
|
|
7.
|
Deak F, Kiss I, Sparks KJ, et al: Complete
amino acid sequence of chicken cartilage link protein deduced from
cDNA clones. Proc Natl Acad Sci USA. 83:3766–3770. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Turley EA, Moore D and Hayden LJ:
Characterization of hyaluronate binding proteins isolated from 3T3
and murine sarcoma virus transformed 3T3 cells. Biochem.
26:2997–3005. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Doege KJ, Sasaki M, Kimura T and Yamada Y:
Complete coding sequence and deduced primary structure of the human
cartilage large aggregating proteoglycan, aggrecan. J Biol Chem.
266:894–902. 1991.
|
|
10.
|
McDevitt CA, Marcelino J and Tucker L:
Interaction of intact type VI collagen with hyaluronan. FEBS Lett.
294:167–170. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ponta H, Sherman L and Herrlich PA: CD44:
from adhesion molecules to signaling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Thorne RF, Legg JW and Isacke CM: The role
of the CD44 transmembrane and cytoplasmic domains in co-ordinating
adhesive and signaling events. J Cell Sci. 117:373–380. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Misra S, Ghatak S and Toole BP: Regulation
of MDR1 expression and drug resistance by a positive feedback loop
involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol
Chem. 280:20310–20315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Bates RC, Edwards NS, Burns GF, et al: A
CD44 survival pathway triggers chemoresistance via Lyn kinase and
phosphoinositide 3-kinase/Akt in colon carcinoma cells. Cancer Res.
61:5275–5283. 2001.PubMed/NCBI
|
|
15.
|
Wang SJ and Bourguignon LY:
Hyaluronan-CD44 promotes phospholipase C-mediated
Ca2+signaling and cisplatin resistance in head and neck
cancer. Arch Otolaryngol Head Neck Surg. 132:19–24. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wang SJ and Bourguignon LY: Hyaluronan and
the interaction between CD44 and epidermal growth factor receptor
in oncogenic signaling and chemotherapy resistance in head and neck
cancer. Arch Otolaryngol Head Neck Surg. 132:771–778. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yang B, Yang BL, Savani RC and Turley EA:
Identification of a common hyaluronan binding motif in the
hyaluronan binding proteins RHAMM, CD44, and link protein. EMBO J.
13:286–296. 1994.PubMed/NCBI
|
|
18.
|
Cohen M, Klein E, Geiger B and Addadi L:
Organization and adhesive properties of hyaluronan pericellular
coat of chondrocytes and epithelial cells. Biophys J. 85:1996–2005.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bourguignon LY, Peyrollier K, Xia W and
Gilad E: Hyaluronan-CD44 interaction activates stem cell marker
Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated
multidrug efflux in breast and ovarian tumor cells. J Biol Chem.
283:17635–17651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Gutova M, Najbauer J, Gevorgyan A, et al:
Identification of uPAR-positive chemoresistant cells in small cell
lung cancer. PLoS One. 2:e2432007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Allouche M, Charrad RS, Bettaieb A, et al:
Ligation of the CD44 adhesion molecule inhibits drug-induced
apoptosis in human myeloid leukemia cells. Blood. 23:1187–1190.
2000.PubMed/NCBI
|