Introduction
Peroxiredoxins (Prdxs) are a family of small
proteins that catalyze the reduction of peroxides using their
conserved Cys residues as catalytical centers (1). Six Prdx isoforms have been found in
mammalian cells, but they are non-redundant antioxidant proteins
(2). The six isoforms of human
Prdxs are located on chromosomes 1, 4, 8, 19 and X, with both
PrdxIII and V located on chromosome 19 (3). The regulation of Prdxs has been
investigated in various types of cancer. The expression of Prdxs,
especially III, IV and V, has been found to be increased in breast
malignancy, suggesting the induction of the expression of Prdxs as
a response to the increased production of reactive oxygen species
(ROS) in carcinoma tissues (4,5).
Moreover, some members of Prdxs are thought to be cancer cell
biomarkers (3). The knockdown of
members of the Prdx family was previously shown to lead to clear
distortion of cell signaling and tumor formation (6–9).
The aim of this study was to validate a previous
finding according to which PrdxV constitutes a tumor marker of the
breast in Sudanese patients (unpublished data), by investigating
the expression pattern of a panel of Prdxs family members in
Sudanese and Chinese patients.
Materials and methods
Patients
A panel of 106 Sudanese breast cancer patients (91
tumors and 79 normal breast tissues, of which 59 were tumor control
pairs) were included in this case-control hospital-based study
(Table I). The tissue samples were
obtained from the El-Zahrawi Medical Center in Khartoum (Sudan) and
the Institute of Endemic Diseases (IEND; University of Khartoum,
Khartoum, Sudan) Tumor Bank. This study was approved by the Ethics
Committee of the IEND and all patients provided informed consent.
The samples were preserved in the form of formalin-fixed
paraffin-embedded (FFPE) tissue blocks.
 | Table IPatient clinicopathological
characteristics of the Sudanese patients. |
Table I
Patient clinicopathological
characteristics of the Sudanese patients.
| Clinicopathological
characteristics | No. (%) |
|---|
| Sample no. | 106 (100) |
| Histological
type | |
| Ductal carcinoma
in situ (DCIS) | 2 (2) |
| Invasive ductal
carcinoma (NOS) | 60 (57) |
| Mixed ductal
carcinoma (DCIS+NOS) | 18 (27) |
| Lobular
carcinoma | 2 (2) |
| Others (e.g.,
papillary and medullary) | 13 (12) |
| Mixed types | 5 (5) |
| Missing data | 5 (5) |
| Total | 105 |
| Mean age
(years) | 46.24 (24–79) |
| Gender | |
| Female | 101 (95.3) |
| Male | 2 (1.9) |
| Missed data | 2 (1.9) |
| Ethnicity | |
| Afro-Asiatic | 41 (39) |
| Nilo-Saharan | 19 (18) |
|
Niger-Kordofanian | 1 (1) |
| Unknown | 44 (42) |
| Nodal
metastasis | |
| Presence | 37 (35.2) |
| Absence | 19 (18.1) |
| Unknown | 46 (46.7) |
| Tumor size
(cm) | |
| from 0 to
<2 | 6 (5.7) |
| from 1 to ≥2 | 70 (66.04) |
| Unknown | 30 (28.3) |
Chinese breast cancer patients
A panel of 31 paired (tumors and controls) tissue
samples from Chinese breast cancer patients (invasive ductal
carcinoma) in the form of tissue arrays (Shanghai Outdo Biotech
Co., Ltd., Shangai, China) were also included in the study.
Immunohistochemistry (IHC)
Both Sudanese and Chinese breast cancer tissue
sections and controls were examined immunohistochemically, for the
following Prdxs antibodies: PrdxI, V and VI (Table II). IHC was performed using the
2-step plus Poly-HRP anti-mouse/rabbit IgG detection system
(biotin-free, anti-mouse/rabbit multivalent) kit (Golden Bridge
International, Everett, WA, USA).
 | Table IIAntibodies used in this study. |
Table II
Antibodies used in this study.
| Protein | Clone | Source | Dilution | Origin |
|---|
| PrdxV | (FL-214) | Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) | 1:500 | Rabbit
polyclonal |
| PrdxI | (ab59538) | Abcam (Cambridge,
MA, USA) | 1:1,000 | Rabbit
polyclonal |
| PrdxVI | (ab59543) | Abcam | 1:1,000 | Rabbit
polyclonal |
| PARP | 9542 | Cell Signaling
Technology, Inc. (Beverly, MA, USA) | 1:1,000 | Rabbit
polyclonal |
| C-Myc | 9E10 | BD Biosciences
(Franklin Lakes, NJ, USA) | 1:1,000 | Mouse
polyclonal |
In situ hybridization (ISH)
To design the PrdxV probe, PCR was performed using
the modified primers (10,11) by the addition of the EcoRI
and BamHI restriction sites (italics) (Invitrogen, Carlsbad,
CA, USA): Prdx5, F, 5′-CGGAATTCATGGCCCCAATCAAGGTGGGAGAT-3′ and R,
5′-CGGGATCCCAGAGCTGTGAGATGATATTGG-3′.
The purified Prd xV gene was cloned using
pcDNA™3.1/myc-His(-)B MCS plasmid (Invitrogen). Plasmids were
prepared using standard methods, as described in a previous study
(12). RNA probes were labeled
with digoxigenin (DIG) using the DIG RNA labeling kit according to
the manufacturer’s instructions (SP6/T7; Roche Diagnostics,
Indianapolis, IN, USA). The optimal concentration of 100
pg/μl was chosen using DIG wash and block buffer according
to the manufacturer’s instructions (Roche Diagnostics). ISH was
performed as described by Breitschopf et al(13). The labeled antisense RNA probe was
diluted (100 pg/μl) in hybridization buffer (12) and a labeled sense RNA probe was
used as the control. The sections were incubated with
alkaline-phosphatase-conjugated anti-DIG antibody, incubated with
NBT/BCIP color reagent (Roche Diagnostics) overnight, and mounted
with a water-soluble mounting medium.
Statistical analysis
The slides were independently examined by two
experienced observers who were blinded to the initial results of
the other observer. Immunoreactivity was allocated a score based on
the percentage of positive tumor cells over total tumor cells
ranging from 0 to 100% and on staining intensity (0, negative; 1,
weak; 2, moderate; and 3, strong). Prdxs immunostaining scores were
as follows: 0, negative or weak staining; and 1, moderate or strong
staining. Clinicopathological parameters for the Sudanese patients
were sub-classified as described in Table I. Descriptive statistics were
calculated using the SPSS 11.5 statistical program. P<0.05
(two-sided test) was considered to indicate a statistically
significant difference.
Results
Immunohistochemistry
The PrdxV level of expression in samples from
Sudanese patients (a panel of 77 tumor and 68 control samples of
which 51 were paired samples) was notably low compared to
previously published studies (4,5).
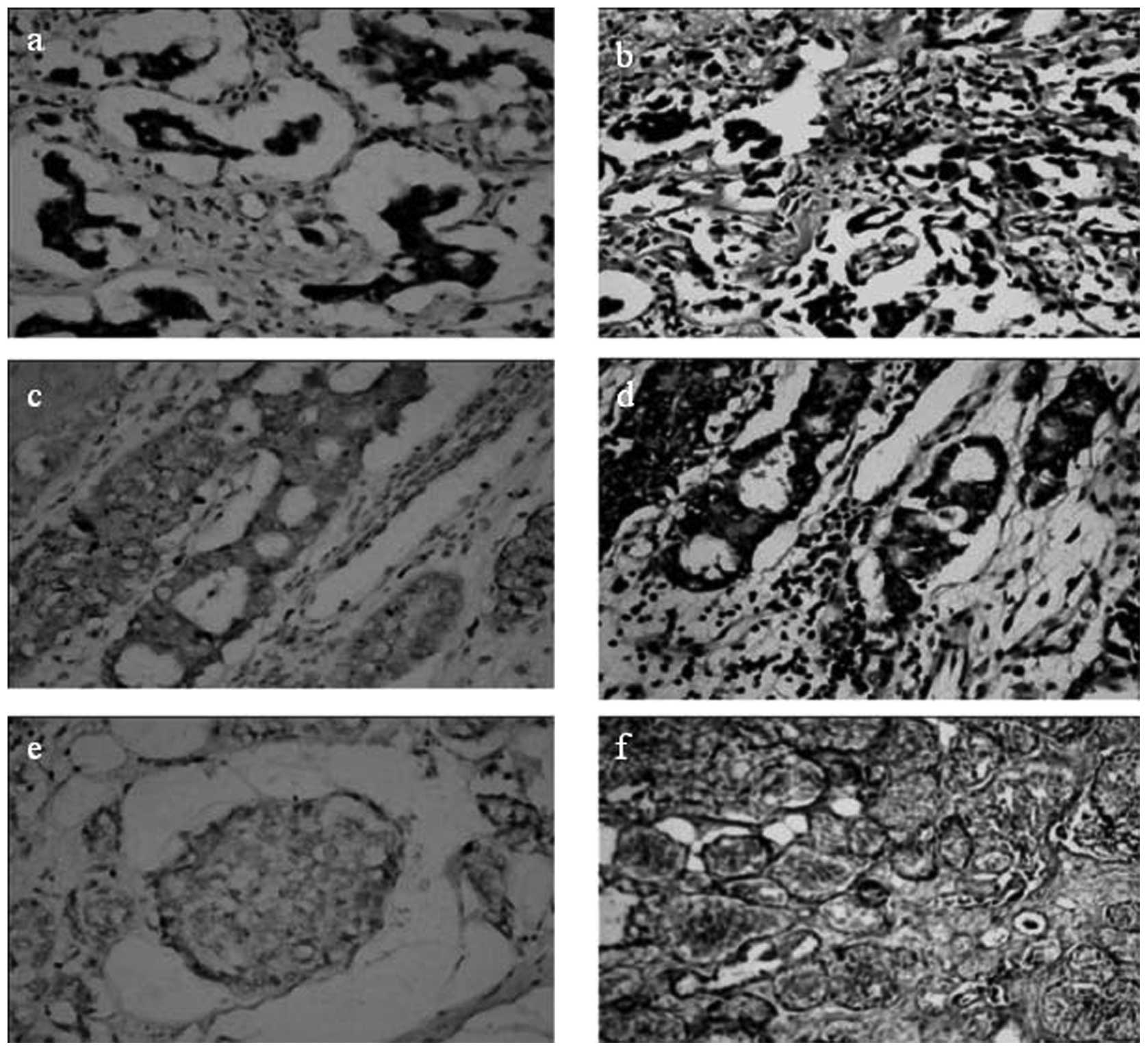
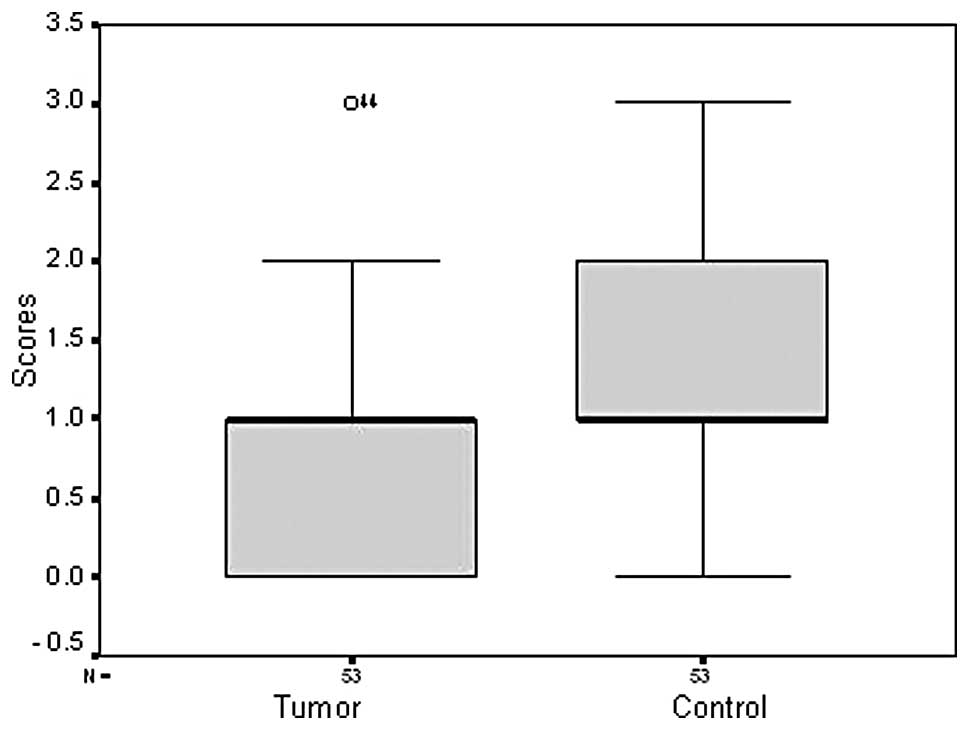
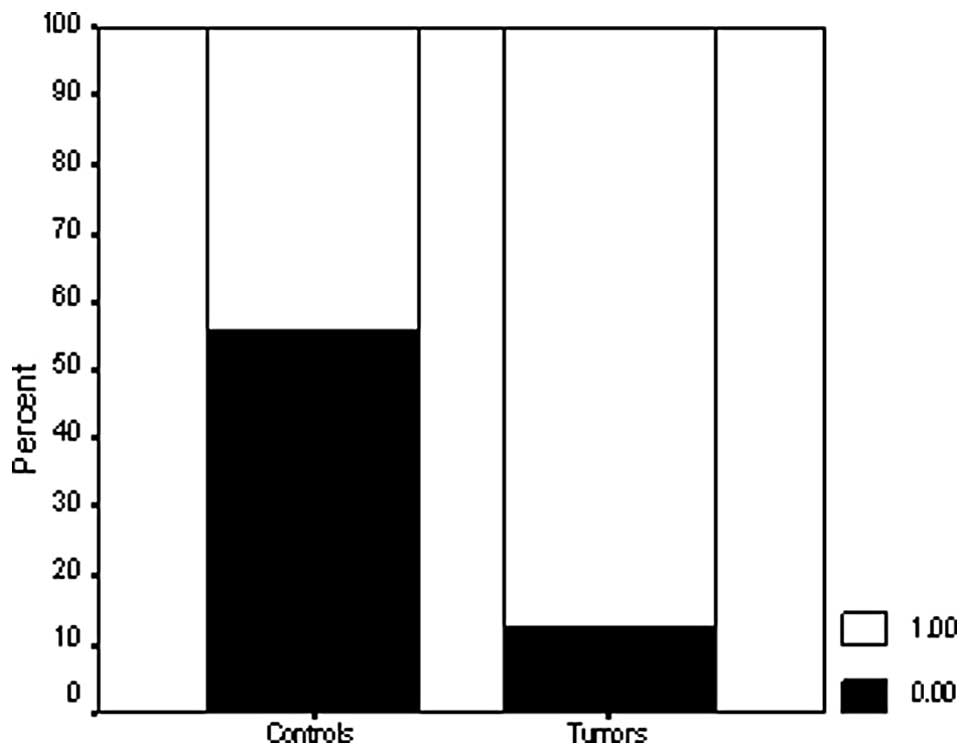
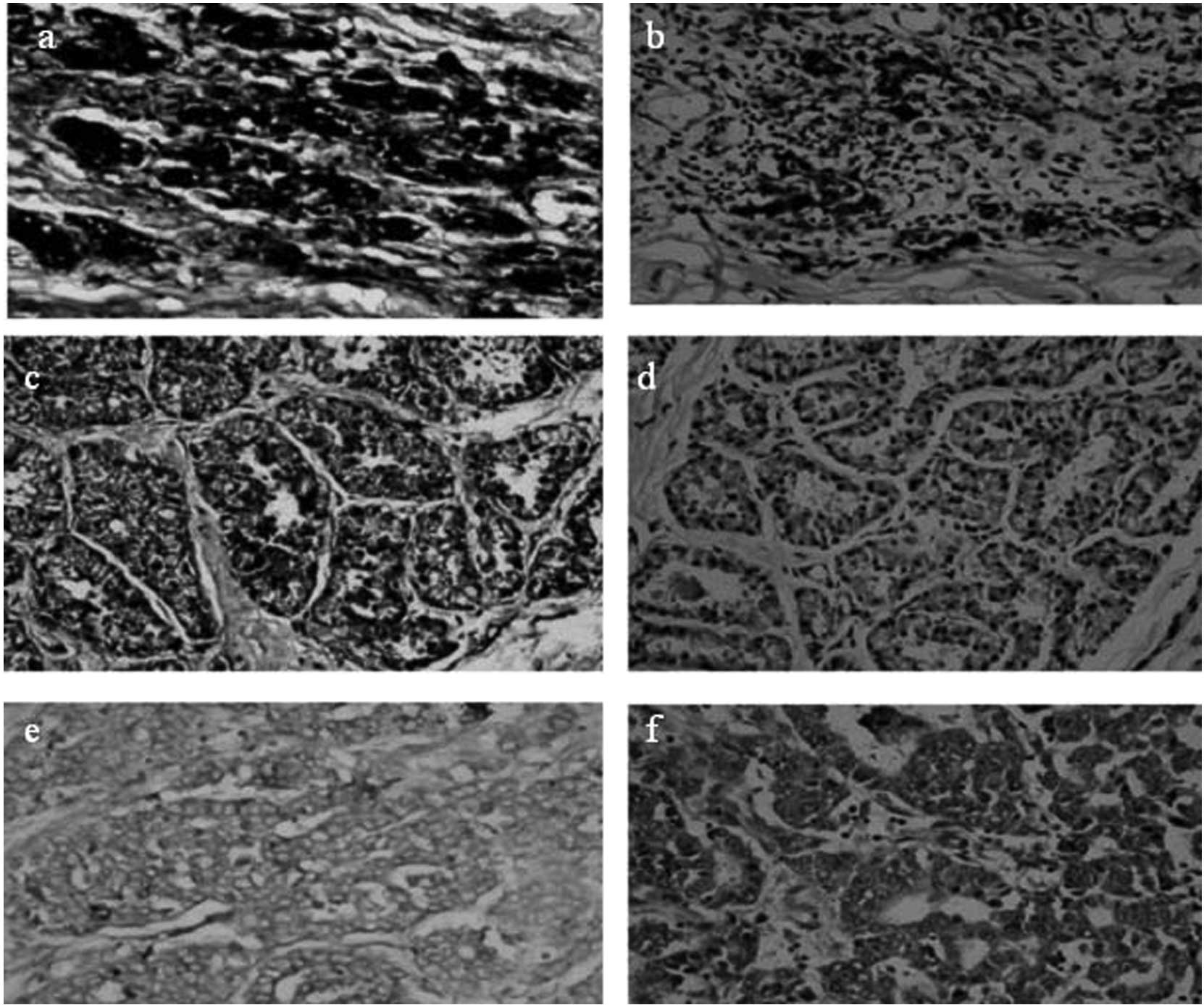
Only 9/77 (11.7%) breast cancer tissue samples were immunoreactive
for the PrdxV antibody, whereas 88.3% of the samples were negative
(Fig. 1). In the control samples,
29/68 (42.6%) were positive for the PrdxV antibody (Fig. 2), indicating a significant
difference between tumors and non-malignant controls (P=0.000)
(Figs. 3 and 4; Table
III). In Chinese samples, PrdxV was found to be predominantly
overexpressed in both tumor and control samples with 24/30 (80%)
tumor samples, and 26/31 (83.9%) control samples being
immunoreactive for the PrdxV antibody (Table III).
 | Table IIIPercentage of Prdx family expression
in Sudanese and Chinese breast cancer patients. |
Table III
Percentage of Prdx family expression
in Sudanese and Chinese breast cancer patients.
| Sudanese
patients | Chinese
patients |
|---|
|
|
|---|
| Antigen | Informative cases,
n | Positive cases n
(%) | Informative
control, n | Positive cases n
(%) | Informative cases,
n | Positive cases n
(%) | Informative
control, n | Positive cases n
(%) |
|---|
| PrdxI
(protein) | 88 | 65 (83) | 62 | 52 (87.1) | 30 | 21 (70) | 27 | 21 (77.8) |
| PrdxV
(protein) | 77 | 9 (11.7)a | 68 | 29 (42.6) | 30 | 24 (80) | 31 | 26 (83.9) |
| PrdxV (mRNA) | 69 | 45 (56.2) | 50 | 41 (87.2) | 31 | 29 (93.5) | 31 | 30 (96.8) |
| PrdxVI
(protein) | 43 | 20 (46.5) | 37 | 21 (56.8) | 30 | 17 (56.7) | 25 | 17 (68) |
Unlike the Sudanese samples, the difference between
PrdxV expression in tumors and controls in Chinese breast cancer
patients was found to be insignificant (P=0.749).
Similarly no correlation was detected between PrdxV
expression and the available pathological parameters, such as
lymphatic invasion (P=1.000), tumor size (P=1.000) and grade
(P=1.000). In addition, there was no correlation with other
demographic parameters, such as age (P=0.412), gender (P=0.108),
ethnicity (P=0.682) and patients’ geographical origin (P=0.686)
(Table IV).
 | Table IVCorrelations between PrdxV
pathological characteristics and other studied Prdxs (Sudanese
samples). |
Table IV
Correlations between PrdxV
pathological characteristics and other studied Prdxs (Sudanese
samples).
|
Characteristics | Negative n (%) | Positive n (%) | P-value |
|---|
| Age (years) | | | |
| ≤46 | 43 (65.2) | 3 (42.9) | 0.412 (F) |
| >46 | 23 (34.8) | 4 (57.1) | |
| Total | 66 (100) | 7 (100) | |
| Gender | | | |
| Female | 74 (100) | 8 (88.9) | 0.108 (F) |
| Male | 0 (0) | 1 (11.1) | |
| Total | 74 (100) | 9 (100) | |
| Lymphatic
invasion | | | |
| Negative | 13 (33.3) | 2 (40) | 1.000 (F) |
| Positive | 26 (66.7) | 3 (60) | |
| Total | 39 (100) | 5 (100) | |
| Tumor size
(cm) | | | |
| from 0 to
<2 | 5 (9.4) | 0 (0) | 1.000 (F) |
| from 1to ≥2 | 48 (90.6) | 7 (100) | |
| Total | 53 (100) | 7 (100) | |
| Grade | | | |
| Low | 1 (2.4) | 0 (0) | 1.000 (F) |
| High | 40 (97.6) | 6 (100) | |
| Total | 41 (100) | 6 (100) | |
| Ethnicity | | | |
| Nilo-Saharan | 15 (35.7) | 1 (20) | 0.682 (F) |
| Afro-Asiatic | 26 (61.9) | 4 (80) | |
|
Niger-Kordofanian | 1 (2.4) | 0 (0) | |
| Total | 42 (100) | 5 (100) | |
| Patients’
geographical origin | | | |
| North | 25 (56.8) | 4 (80) | 0.686 (F) |
| East | 1 (2.3) | 0 (0) | |
| West | 11 (25) | 0 (0) | |
| Center | 5 (11.4) | 1 (20) | |
| South | 2 (4.5) | 0 (0) | |
| Breast cancer
type | | | |
| NOS | 41 (56.2) | 6 (75) | 0.403 (F) |
| DCIS | 0 (0) | 0 (0) | |
| LCIS | 1 (1.4) | 0 (0) | |
| NOS+DCIS | 14 (19.2) | 2 (25) | |
| Other types | 12 (16.4) | 0 (0) | |
| Mixed types | 5 (6.8) | 0 (0) | |
| Total | 73 (100) | 8 (100) | |
| ISH | | | |
| Negative | 22 (95.7) | 38 (86.4) | 0.000 (Chi) |
| Positive | 1 (4.3) | 6 (13.6) | |
| Total | 23 (100) | 44 (100) | |
| PrdxI | | | |
| Negative | 16 (23.2) | 0 (0) | 0.000 (Chi) |
| Positive | 53 (76.8) | 5 (100) | |
| Total | 69 (100) | 5 (100) | |
| PrdxVI | | | |
| Negative | 20 (55.6) | 1 (33.3) | 0.000 (Chi) |
| Positive | 16 (44.4) | 2 (66.7) | |
| Total | 36 (100) | 3 (100) | |
Expression of PrdxI and VI protein was also examined
immunohistochemically in Sudanese and Chinese tissue samples and
was found to be overexpressed in both tumors and controls in
Sudanese and Chinese breast tissue samples (Tables III and VI, respectively). The difference between
PrdxI and VI protein expression in tumors and controls in Sudanese
and Chinese samples was statistically examined and found to be
insignificant. No significant correlation was observed between the
studied clinicopathological parameters and PrdxI and VI protein
expression in both Sudanese and Chinese breast carcinomas (Tables V and VI, respectively).
 | Table VICorrelations of PrdxVI with
pathological characteristics (Sudanese samples). |
Table VI
Correlations of PrdxVI with
pathological characteristics (Sudanese samples).
|
Characteristics | Negative n (%) | Positive n (%) | P-value |
|---|
| Age (years) | | | |
| ≤46 | 43 (65.2) | 3 (42.9) | 0.412 (F) |
| >46 | 23 (34.8) | 4 (57.1) | |
| Total | 66 (100) | 7 (100) | |
| Gender | | | |
| Female | 74 (100) | 8 (88.9) | 0.108 (F) |
| Male | 0 (0) | 1 (11.1) | |
| Total | 74 (100) | 9 (100) | |
| Lymphatic
invasion | | | |
| Negative | 13 (33.3) | 2 (40) | 1.000 (F) |
| Positive | 26 (66.7) | 3 (60) | |
| Total | 39 (100) | 5 (100) | |
| Tumor size
(cm) | | | |
| from 0 to
<2 | 5 (9.4) | 0 (0) | 1.000 (F) |
| from 1 to ≥2 | 48 (90.6) | 7 (100) | |
| Total | 53 (100) | 7 (100) | |
| Grade | | | |
| Low | 1 (2.4) | 0 (0) | 1.000 (F) |
| High | 40 (97.6) | 6 (100) | |
| Total | 41 (100) | 6 (100) | |
| Breast cancer
type | | | |
| NOS | 13 (56.5) | 10(52.6) | 0.056 (F) |
| DCIS | 0 (0) | 0 (0) | |
| LCIS | 0 (0) | 0 (0) | |
| NOS+DCIS | 5 (21.7) | 4 (21.1) | |
| Other types | 3 (13) | 4 (21.1) | |
| Mixed types | 2 (8.7) | 1 (5.3) | |
| Total | 23(100) | 19 (100) | |
| Ethnicity | | | |
| Nilo-Saharan | 15 (35.7) | 1 (20) | 0.682 (F) |
| Afro-Asiatic | 26 (61.9) | 4 (80) | |
|
Niger-Kordofanian | 1 (2.4) | 0 (0) | |
| Total | 42 (100) | 5 (100) | |
| Patients’
geographical origin | | | |
| North | 25 (56.8) | 4 (80) | 0.960 (F) |
| East | 1 (2.3) | 0 (0) | |
| West | 11 (25) | 0 (0) | |
| Center | 5 (11.4) | 1 (20) | |
| South | 2 (4.5) | 0 (0) | |
| Total | 44 (100) | 5 (100) | |
 | Table VCorrelations of PrdxI with
pathological characteristics (Sudanese samples). |
Table V
Correlations of PrdxI with
pathological characteristics (Sudanese samples).
|
Characteristics | Negative n (%) | Positive n (%) | P-value |
|---|
| Age (years) | | | |
| ≤46 | 43 (65.2) | 3 (42.9) | 0.412 (F) |
| >46 | 23 (34.8) | 4 (57.1) | |
| Total | 66 (100) | 7 (100) | |
| Gender | | | |
| Female | 74 (100) | 8 (88.9) | 0.108 (F) |
| Male | 0 (0) | 1 (11.1) | |
| Total | 74 (100) | 9 (100) | |
| Lymphatic
invasion | | | |
| Negative | 13 (33.3) | 2 (40) | 1.000 (F) |
| Positive | 26 (66.7) | 3 (60) | |
| Total | 39 (100) | 5 (100) | |
| Tumor size
(cm) | | | |
| from 0 to
<2 | 5 (9.4) | 0 (0) | 1.000 (F) |
| from 1 to ≥2 | 48 (90.6) | 7 (100) | |
| Total | 53 (100) | 7 (100) | |
| Grade | | | |
| Low | 1 (2.4) | 0 (0) | 1.000 (F) |
| High | 40 (97.6) | 6 (100) | |
| Total | 41 (100) | 6 (100) | |
| Breast cancer
type | | | |
| NOS | 8 (50) | 37(59.7) | 0.056 (F) |
| DCIS | 0 (0) | 0 (0) | |
| LCIS | 0 (0) | 1 (1.6) | |
| NOS+DCIS | 2 (12.5) | 14 (22.6) | |
| Other types | 6 (37.5) | 5 (8.1) | |
| Mixed types | 0 (6.8) | 5 (8.1) | |
| Total | 16 (100) | 62 (100) | |
| Ethnicity | | | |
| Nilo-Saharan | 15 (35.7) | 1 (20) | 0.682 (F) |
| Afro-Asiatic | 26 (61.9) | 4 (80) | |
|
Niger-Kordofanian | 1 (2.4) | 0 (0) | |
| Total | 42 (100) | 5 (100) | |
| Patients’
geographical origin | | | |
| North | 25 (56.8) | 4 (80) | 0.686 (F) |
| East | 1 (2.3) | 0 (0) | |
| West | 11 (25) | 0 (0) | |
| Center | 5 (11.4) | 1 (20) | |
| South | 2 (4.5) | 0 (0) | |
| Total | 44 (100) | 5 (100) | |
In situ hybridization
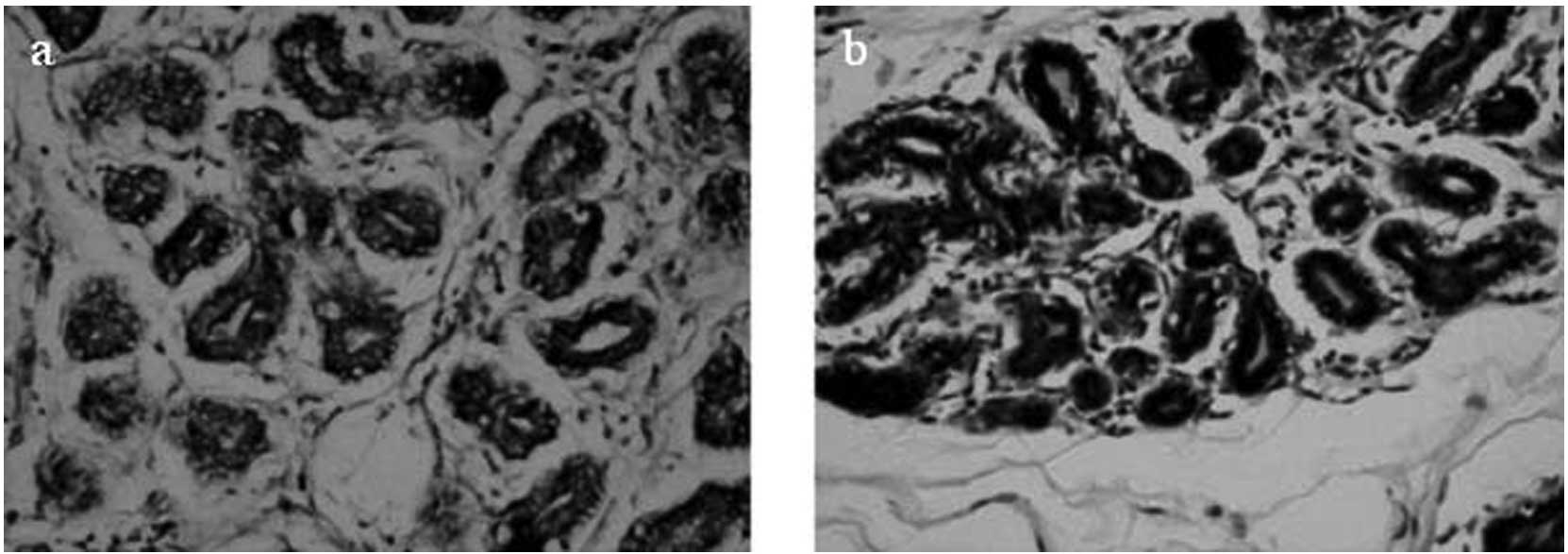
The mRNA expression level was examined only for
PrdxV using ISH to verify whether the difference in expression
between tumors and controls is at the protein level only or present
at the mRNA level as well. In Sudanese breast tissue samples, a
total of 69 tumor samples and 50 controls were examined, of which
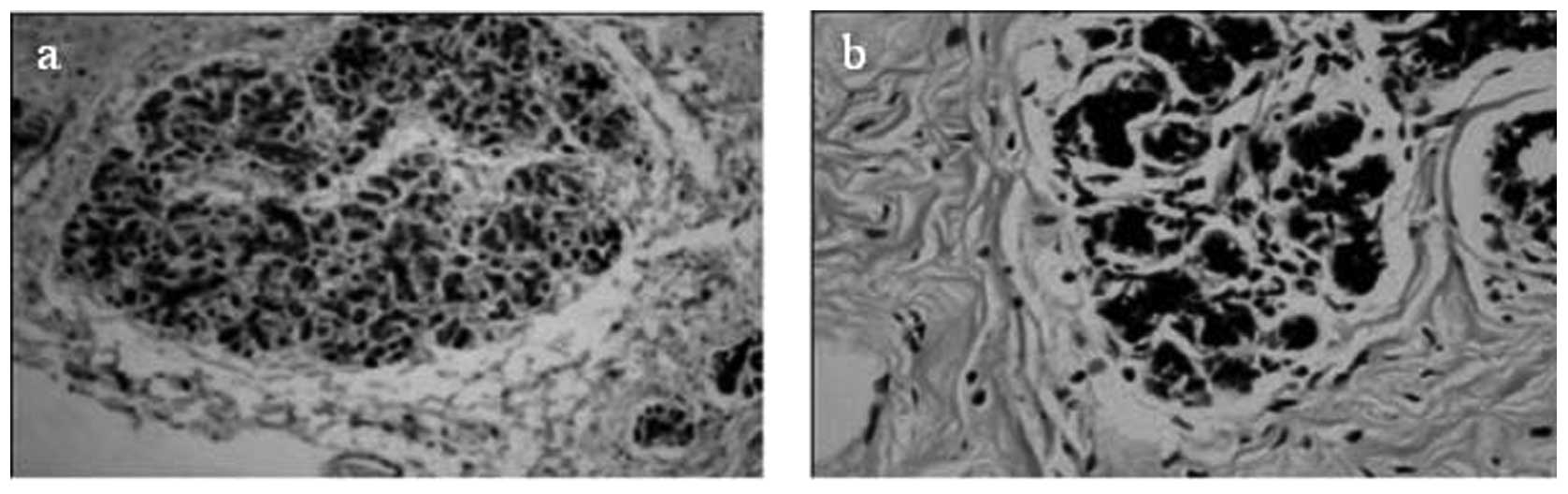
45/69 (65.2%) tumor samples (Fig.
5) and 41/50 controls (87.2%) (Fig. 6) were positive. The difference in
expression levels between tumors and controls was found to be
significant (P= 0.044) (Table
III).
In Chinese samples, the mRNA level of PrdxV
correlations expression was also studied, using ISH. Tumor samples
(29/31) (93.5%) were positive, as were 30/31 (96.8%) control
samples (Table III). Unlike
Sudanese samples, no significant difference was found between tumor
and control samples (P=1.000).
Discussion
In the present study, a panel of Sudanese breast
cancer tissue samples and healthy controls were investigated.
Tissue sections were examined immunohistochemically to assess the
PrdxV protein level of expression. The PrdxV mRNA expression was
also evaluated using ISH. The bold score of PrdxV expression is
representative of the finding of a preliminary proteomic study
suggesting that PrdxV is differentially expressed in Sudanese
breast cancer tissues as compared to healthy controls (unpublished
data) (Table III). Various Prdx
family members (PrdxI and VI) were also examined to ensure the
specificity of PrdxV, mainly as a tumor marker among other family
members. Additionally, to determine whether the PrdxV mode of
expression is universal or limited to Sudanese breast cancer
patients, the same experimental design was implemented in a panel
of Chinese breast carcinoma samples and controls, and the two
populations were compared.
Of the various Prdx family members, PrdxV was the
only one that was significantly downregulated in tumor samples
obtained from Sudanese breast cancer patients. At the protein
level, only a few tumor samples were immunoreactive for PrdxV, only
9/77 (11.7%) were positive (P<0.0001), while 29/68 (47%) of the
controls were immunoreactive (P=0.225). Based on these results, it
appears that the PrdxV protein is not abundantly in present
Sudanese neoplastic breast tissues, likely due to the fact that
PrdxV may have a different role in these cells. This finding is
contradictory to previous findings by Karihtala et
al(5), where 79.8% of the
studied cases were positive for PrdxV antibody, suggesting that a
larger set of controls (n=68) was investigated in this study
compared to that by Karihtala et al(5) where only three controls were examined
immunohistochemically. The finding that PrdxV is downregulated in
Sudanese breast cancer samples is also inconsistent with results
obtained in a panel of Chinese breast carcinoma samples and
controls, where PrdxV protein was found to be overexpressed in both
tumors (80%) and controls (83.9%). This finding is also
inconsistent with various Prdx family members examined (PrdxI and
VI) in both Sudanese and Chinese tumor tissues and controls
(Table III), suggesting the
expression discrepancy to be restricted to the PrdxV protein of
Sudanese patients only.
To investigate whether PrdxV gene expression
loss, assessed by IHC, occurs at the protein level or at an earlier
stage, such as at the transcription level, the mRNA mode of
expression was investigated using ISH and was found to be
overexpressed in both tumors and controls, with 56.2% of the
studied tumors being immunoreactive to PrdxV antibody (P=0.011),
and 87.2% of controls being positive (P<0.0001). PrdxV mRNA
expression was also found to be significantly different between
tumors and controls (P=0.044). In the Chinese cases, PrdxV mRNA was
found to be overexpressed in both tumors (93.5%) and controls
(96.8%), with no significant difference being identified in the
expression between tumors and controls. ISH results assessing PrdxV
mRNA expression levels in this study were in accordance with
previous findings (14) assessing
mRNA expression levels for all members of the Prdx and thioredoxin
(Trx) families. PrdxV mRNA expression levels were found to be
upregulated in breast cancer compared to normal breast tissues
(14), and were in accordance with
the immunohistochemical findings of PrdxV in Sudanese samples
regarding the difference between tumors and controls. However, the
difference was more apparent and statistically significant at the
protein level, suggesting a post-translational modification. The
regulation of Prdx activity occurs at the gene expression level and
by post-translational protein modification and has received
considerable attention (15–18).
An example of gene expression loss and its effect on tumorigenesis
is the loss of E-cadherin expression in lobular carcinomas of the
breast (19–21).
The PrdxV protein expression loss in Sudanese breast
cancer patients may have two consequences of antagonistic nature
during tumorigenesis. The first one is the expected poor prognosis
of tumors due to loss of PrdxV function which protects tissues from
harmful ROS, which are usually considered to have carcinogenic
potential and promote invasiveness (22,23).
Prdx isoforms are non-redundant antioxidant proteins (2), since previous studies have shown that
the knockdown of members of the Prdx family led to the distortion
of cell signaling and tumor formation (6,7,9).
Therefore, the down-regulation of PrdxV in Sudanese breast cancer
patients is expected to yield cells more prone to oxidative stress
and its accompanying DNA damage, leading to a poorer prognosis of
Sudanese breast cancer patients. The second consequence of the
PrdxV protein expression loss in Sudanese breast cancer patients is
correlated with the absence of the PrdxV anti-apoptotic function,
which is expected to induce breast cancer tissue sensitivity to
chemo- and radiotherapies. PrdxV has been previously shown to have
a protective role against oxidative stress and to lead to apoptosis
(24–26).
Clinicopathological data were obtained for most of
the studied cases, correlations were examined comparing the Prxds
examined in the present study to each other, to available
demographic data including age, gender and ethnicity, as well as to
the available pathological parameters such as histological types,
nodal metastasis and tumor size, which are also known prognostic
markers of breast cancer. No statistically significant correlation
was identified, whereas Karihtala et al(5) detected significant associations
between PrdxV overexpression and larger tumor size, lymph node
metastases, and poor differentiation of tumors. The finding in this
study may not constitute a conclusion regarding the correlation
between PrdxV expression and pathological parameters, since those
parameters are not normally distributed, as most breast cancer
patients present in Sudan, present with large tumors, high grades,
and positive metastatic nodes in the majority of cases. This
observation has been previously noted in Sudanese (27,28),
as well as in African breast cancer patients (29).
The most important finding in this study is the
marked downregulation of PrdxV in Sudanese breast cancer patients,
suggesting that the molecule be regarded as a tumor marker.
Numerous markers were previously identified with only a limited
number of these markers being accepted for routine clinical use,
such as HER2, ER and PR (30). Although this study suggests that
PrdxV be regarded as a tumor marker of population specificity, it
should be taken into consideration that breast cancer in individual
patients may differ widely from one another in natural history and
response to treatment (31).
Population differences in breast cancer characteristics were
previously described in terms of pathological parameters (27,31,32),
and in terms of the identification of mutations, intronic variant
sequences and unclassified variants, as well as variated copy
numbers, reporting a role in breast cancer susceptibility that
remains to be clarified. For instance, mutations in the
predisposition genes BRCA1 and BRCA2, known to be
associated with hereditary breast cancer, were studied in different
populations such as Sudanese (33,34),
Tunisian (35), Indian (36) and Slovak (37), where population-specific genetic
disorders were observed. Similarly, the Mspl polymorphism in
the 3′-non-coding region of the CYP1A1 gene was associated
with breast cancer in African-American but not in Caucasian women
(38).
In conclusion, the downregulation of PrdxV in
Sudanese breast cancer patients is suggested as a tumor marker of
population specificity. However, additional studies are needed to
investigate the applicability of PrdxV as a tumor marker in
Sudanese breast cancer patients complementing, but not replacing,
the traditional diagnostic and prognostic markers. Additionally,
further investigation is required to determine ways of
incorporating this suggested marker into routine radio- and/or
chemotherapy.
Abbreviations:
|
Prdxs
|
peroxiredoxins
|
|
PrdxV
|
peroxiredoxin V
|
|
IEND
|
Institute of Endemic Diseases
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
IHC
|
immunohistochemistry
|
|
ISH
|
in situ hybridization
|
Acknowledgements
This study was funded by the Third
World Organization for Women in Science (TWOWS) and the Swedish
International Development Cooperation Agency (SIDA).
References
|
1.
|
Greenberg JT and Demple B: Overproduction
of peroxide-scavenging enzymes in Escherichia colisuppresses
spontaneous mutagenesis and sensitivity to redox-cycling agents in
oxyR-mutants. Embo J. 7:2611–2617. 1988.PubMed/NCBI
|
|
2.
|
Dietz KJ: The dual function of plant
peroxiredoxins in antioxidant defence and redox signaling. Subcell
Biochem. 44:267–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zhang B, Wang Y and Su Y: Peroxiredoxins,
a novel target in cancer radiotherapy. Cancer Lett. 286:154–160.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Noh DY, Ahn SJ, Lee RA, Kim SW, Park IA
and Chae HZ: Overexpression of peroxiredoxin in human breast
cancer. Anticancer Res. 21:2085–2090. 2001.PubMed/NCBI
|
|
5.
|
Karihtala P, Mantyniemi A, Kang SW,
Kinnula VL and Soini Y: Peroxiredoxins in breast carcinoma. Clin
Cancer Res. 9:3418–3424. 2003.PubMed/NCBI
|
|
6.
|
Neumann CA, Krause DS, Carman CV, Das S,
Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH and Van
Etten RA: Essential role for the peroxiredoxin Prdx1 in erythrocyte
antioxidant defence and tumour suppression. Nature. 424:561–565.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lee TH, Kim SU, Yu SL, Kim SH, Park DS,
Moon HB, Dho SH, Kwon KS, Kwon HJ, Han YH, et al: Peroxiredoxin II
is essential for sustaining life span of erythrocytes in mice.
Blood. 101:5033–5038. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang X, Phelan SA, Forsman-Semb K, Taylor
EF, Petros C, Brown A, Lerner CP and Paigen B: Mice with targeted
mutation of peroxiredoxin 6 develop normally but are susceptible to
oxidative stress. J Biol Chem. 278:25179–25190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
De Simoni S, Goemaere J and Knoops B:
Silencing of peroxiredoxin 3 and peroxiredoxin 5 reveals the role
of mitochondrial peroxiredoxins in the protection of human
neuroblastoma SH-SY5Y cells toward MPP+. Neurosci Lett.
433:219–224. 2008.PubMed/NCBI
|
|
10.
|
Seo MS, Kang SW, Kim K, Baines IC, Lee TH
and Rhee SG: Identification of a new type of mammalian
peroxiredoxin that forms an intramolecular disulfide as a reaction
intermediate. J Biol Chem. 275:20346–20354. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kropotov AV, Grudinkin PS, Pleskach NM,
Gavrilov BA, Tomilin NV and Zhivotovsky B: Downregulation of
peroxiredoxin V stimulates formation of etoposide-induced
double-strand DNA breaks. FEBS Lett. 572:75–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sambrook J, Fritsch EF and Maniatis T:
Molecular Cloning: A Laboratory Manual. Cold Spring Harbor
Laboratory Press; New York: pp. 1989
|
|
13.
|
Breitschopf H, Suchanek G, Gould RM,
Colman DR and Lassmann H: In situ hybridization with
digoxigenin-labeled probes: sensitive and reliable detection method
applied to myelinating rat brain. Acta Neuropathol. 84:581–587.
1992. View Article : Google Scholar
|
|
14.
|
Cha MK, Suh KH and Kim IH: Overexpression
of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J
Exp Clin Cancer Res. 28:932009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Immenschuh S and Baumgart-Vogt E:
Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid
Redox Signal. 7:768–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Chang TS, Jeong W, Choi SY, Yu S, Kang SW
and Rhee SG: Regulation of peroxiredoxin I activity by
Cdc2-mediated phosphorylation. J Biol Chem. 277:25370–25376. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wagner E, Luche S, Penna L, Chevallet M,
Van Dorsselaer A, Leize-Wagner E and Rabilloud T: A method for
detection of overoxidation of cysteines: peroxiredoxins are
oxidized in vivo at the active-site cysteine during oxidative
stress. Biochem J. 366:777–785. 2002.PubMed/NCBI
|
|
18.
|
Lehtonen S: Localization and regulation of
peroxiredoxins in human lung and lung diseases. Oulun Yliopisto;
Oulu: 2005
|
|
19.
|
Moll R, Mitze M, Frixen UH and Birchmeier
W: Differential loss of E-cadherin expression in infiltrating
ductal and lobular breast carcinomas. Am J Pathol. 143:1731–1742.
1993.PubMed/NCBI
|
|
20.
|
Vos CB, Cleton-Jansen AM, Berx G, de Leeuw
WJ, ter Haar NT, van Roy F, Cornelisse CJ, Peterse JL and van de
Vijver MJ: E-cadherin inactivation in lobular carcinoma in situ of
the breast: an early event in tumorigenesis. Br J Cancer.
76:1131–1133. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Gown AM: Genogenic immunohistochemistry: a
new era in diagnostic immunohistochemistry. Curr Diagn Pathol.
8:193–200. 2002. View Article : Google Scholar
|
|
22.
|
Wood ZA, Poole LB and Karplus PA:
Peroxiredoxin evolution and the regulation of hydrogen peroxide
signaling. Science. 300:650–653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rhee SG, Jeong W, Chang TS and Woo HA:
Sulfiredoxin, the cysteine sulfinic acid reductase specific to
2-Cys peroxiredoxin: its discovery, mechanism of action, and
biological significance. Kidney Int Suppl. 106:S3–S8. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zhou Y, Kok KH, Chun AC, Wong CM, Wu HW,
Lin MC, Fung PC, Kung H and Jin DY: Mouse peroxiredoxin V is a
thioredoxin peroxidase that inhibits p53-induced apoptosis. Biochem
Biophys Res Commun. 268:921–927. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Banmeyer I, Marchand C, Verhaeghe C, Vucic
B, Rees JF and Knoops B: Overexpression of human peroxiredoxin 5 in
subcellular compartments of Chinese hamster ovary cells: effects on
cytotoxicity and DNA damage caused by peroxides. Free Radic Biol
Med. 36:65–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kropotov A, Serikov V, Suh J, Smirnova A,
Bashkirov V, Zhivotovsky B and Tomilin N: Constitutive expression
of the human peroxiredoxin V gene contributes to protection of the
genome from oxidative DNA lesions and to suppression of
transcription of noncoding DNA. FEBS J. 273:2607–2617. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Awadelkarim KD, Arizzi C, Elamin EO, Hamad
HM, De Blasio P, Mekki SO, Osman I, Biunno I, Elwali NE,
Mariani-Costantini R, et al: Pathological, clinical and prognostic
characteristics of breast cancer in Central Sudan versus Northern
Italy: implications for breast cancer in Africa. Histopathology.
52:445–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Elgaili EM, Abuidris DO, Rahman M,
Michalek AM and Mohammed SI: Breast cancer burden in central Sudan.
Int J Womens Health. 2:77–82. 2010.
|
|
29.
|
Khaled HM: Breast cancer at diagnosis in
women of Africa and the Middle East. Breast Cancer in Women of
African Descent. Williams CKO, Olopade OI and Falkson CI: Springer;
Dordrecht, The Netherlands: pp. 1975–2004. 2006
|
|
30.
|
Dressler LG, Berry DA, Broadwater G, Cowan
D, Cox K, Griffin S, Miller A, Tse J, Novotny D, Persons DL, et al:
Comparison of HER2 status by fluorescence in situ hybridization and
immunohistochemistry to predict benefit from dose escalation of
adjuvant doxorubicin-based therapy in node-positive breast cancer
patients. J Clin Oncol. 23:4287–4297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Carlson RW and Stockdale FE: The clinical
biology of breast cancer. Annu Rev Med. 39:453–464. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Ijaduola TG and Smith EB: Pattern of
breast cancer among white-American, African-American, and
nonimmigrant west-African women. J Natl Med Assoc. 90:547–551.
1998.PubMed/NCBI
|
|
33.
|
Masri MA, Abdel Seed NM, Fahal AH, Romano
M, Baralle F, El Hassam AM and Ibrahim ME: Minor role for BRCA2
(exon11) and p53 (exon 5–9) among Sudanese breast cancer patients.
Breast Cancer Res Treat. 71:145–147. 2002.PubMed/NCBI
|
|
34.
|
Awadelkarim KD, Aceto G, Veschi S, Elhaj
A, Morgano A, Mohamedani AA, Eltayeb EA, Abuidris D, Di Gioacchino
M, Battista P, et al: BRCA1 and BRCA2 status in a Central Sudanese
series of breast cancer patients: interactions with genetic, ethnic
and reproductive factors. Breast Cancer Res Treat. 102:189–199.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Mahfoudh W, Bouaouina N, Ahmed SB, Gabbouj
S, Shan J, Mathew R, Uhrhammer N, Bignon YJ, Troudi W, Elgaaied AB,
et al: Hereditary breast cancer in Middle Eastern and North African
(MENA) populations: identification of novel, recurrent and founder
BRCA1 mutations in the Tunisian population. Mol Biol Rep.
39:1037–1046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Singh AK, Pandey A, Tewari M, Shukla HS
and Pandey HP: Epigenetic silencing of BRCA1 gene associated with
demographic and pathologic factors in sporadic breast cancer: a
study of an Indian population. Eur J Cancer Prev. 20:478–483. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Konecny M, Milly M, Zavodna K, Weismanova
E, Gregorova J, Mlkva I, Ilencikova D, Kausitz J and Bartosova Z:
Comprehensive genetic characterization of hereditary breast/ovarian
cancer families from Slovakia. Breast Cancer Res Treat.
126:119–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Zhu K, Hunter S, Payne-Wilks K, Sutcliffe
C, Bentley C, Roland CL and Williams SM: Potential differences in
breast cancer risk factors based on CYP1A1 MspI and
African-American-specific genotypes. Ethn Dis. 16:207–215.
2006.PubMed/NCBI
|