Introduction
Lung cancer has been the leading cause of mortality
from cancer in Japan since 1998, with ∼50,000 deaths annually.
Anti-smoking measures as a primary prevention strategy, early
diagnosis through health-check campaigns, as well as advances in
surgical therapy, chemotherapy and radiation therapy have improved
overall prognosis. However, the 5-year survival rate remains at
only ∼13%. Compared with other types of cancer, sufficient lung
cancer therapeutic results have yet to be achieved. Lung cancer is
frequently diagnosed in advanced stages, thus, the primary
treatment mainly comprises platinum combination chemotherapy.
Carboplatin + paclitaxel combination chemotherapy (CbPac therapy)
is a typical regimen for non-small cell lung cancer (NSCLC), and is
widely used in clinical practice.
The occurrence, progression and metastasis of cancer
involve various gene and protein abnormalities. In lung cancer,
mutations of the epidermal growth factor receptor (EGFR) and KRAS
genes as well as abnormalities in protein expression have been
previously reported (1–3). Recently, the correlation of these
gene mutations and protein expression abnormalities with the
therapeutic effects has been extensively studied, and EGFR gene
mutation was identified as a predictive factor of the therapeutic
effect of EGFR tyrosine kinase inhibitors (4,5).
Thymidylate synthase (TS) is a key enzyme in DNA
synthesis and cell growth that has been suggested to be involved in
malignancy (6–8). Moreover, it is a main target protein
of antimetabolites, such as the anticancer agents pemetrexed (Pem)
(9) and S-1 (10), demonstrating clinical efficacy in
NSCLC. In colon (11), breast
(12) and pancreatic cancer
(13), an association between TS
expression in cancer tissue, antitumor effects of chemotherapy and
prognosis has been suggested. In lung cancer, a correlation between
TS expression and prognosis has been suggested in early cancer
(14). A limited number of studies
have examined TS expression in advanced lung cancer, however, its
impact on clinical effects remains to be determined. In particular,
whether TS expression in cancer tissue is involved in the efficacy
of CbPac therapy and prognosis remains to be elucidated. The aim of
this study was to examine TS expression in cancer tissues obtained
from patients with advanced lung cancer, and investigate the
correlation between the expression rate and therapeutic effects and
prognosis.
Patients and methods
Patients
In total, 120 patients diagnosed with lung cancer at
Nihon University Hospital (Tokyo, Japan) between June 2004 and
December 2010 were included in this study. Cancer tissue specimens
were obtained from the included patients prior to treatment. The
method of this study was approved by the ethics committee of Nihon
University School of Medicine. Written informed consent was
obtained from each subject. Cancer tissue specimens were collected
by surgical procedure or bronchofiberscopic biopsy, then fixed in
formalin and embedded in paraffin. Immunostaining was performed to
examine the expression of TS protein. Patient background
information is provided in Table
I. The patients comprised 78 males and 42 females (mean age,
65.7 years). Additionally, there were 81 patients with
adenocarcinoma, 17 with squamous cell carcinoma, 12 with non-small
cell carcinoma, 7 with large cell carcinoma and 3 with small cell
carcinoma. Eleven patients were positive for the EGFR gene
mutation; performance status (PS) was 0–1 in 103 patients; the
disease stage was IIIB or IV in 100 patients; and there were 29
non-smokers. The primary treatment for 85 patients (71%) was
chemotherapy alone, and out of these 85 patients, 50 were
administered CbPac combined chemotherapy (Table I).
 | Table IPatient characteristics and treatment
methods. |
Table I
Patient characteristics and treatment
methods.
| Characteristics | Value, n (%) |
|---|
| Age (years) | |
| Mean (range) | 65.7 (23–85) |
| Gender | |
| Male | 78 (65.0) |
| Female | 42 (35.0) |
| Histology | |
| Adenocarcinoma | 81 (67.5) |
| Squamous cell
carcinoma | 17 (14.2) |
| Non-small cell
carcinoma | 12 (10.0) |
| Large cell
carcinoma | 7 (5.8) |
| Small cell
carcinoma | 3 (2.5) |
| EGFR mutation | |
| Positive | 11 (9.2) |
| Negative | 58 (48.3) |
| Unknown | 51 (42.5) |
| ECOG performance
status | |
| 0 | 32 (26.7) |
| 1 | 71 (59.2) |
| 2 | 12 (10.0) |
| 3,4 | 5 (4.2) |
| Stage of disease | |
| I, II | 8 (6.7) |
| IIIA | 12 (10.0) |
| IIIB | 27 (22.5) |
| IV | 73 (60.8) |
| Smoking status | |
| Former/current
smoker | 89 (74.2) |
| Non-smoker | 29 (24.2) |
| Unknown | 2 (1.7) |
| TS protein
expession | |
| High | 66 (55.0) |
| Low | 54 (45.0) |
| Treatment | |
| Operation | 7 (5.8) |
| Thoracic
radiotherapy | 2 (1.7) |
| Chemotherapy plus
radiotherapy | 23 (19.2) |
| Chemotherapy | |
| CbPac | 50 (41.7) |
| Gemcitabine | 2 (1.7) |
| Cisplatin +
S-1 | 1 (0.8) |
| Docetaxel | 1 (0.8) |
| Gefitinib | 3 (2.5) |
| Carboplatin +
Irinotecan | 2 (1.7) |
| Carboplatin +
gemcitabine | 2 (1.7) |
| Carboplatin +
PMT | 14 (11.7) |
| Cisplatin +
VNR | 1 (0.8) |
| Irinotecan | 1 (0.8) |
| PMT | 1 (0.8) |
| Chemotherapy | |
| TS1 | 4 (3.3) |
| VNR | 3 (2.5) |
| Others | 3 (2.5) |
TS immunostaining
Using the paraffin block of lung cancer tissue
collected prior to treatment, the expression of TS protein was
examined immunohistochemically. The paraffin block was cut into
10-μm sections and stained using immunostaining methods. The
sections were then deparaffinized by being treated with 100% xylene
three times for 2 min each, immersed in 99, 90 and 70% ethanol for
1 min each, washed with running water for 3 min and then reacted in
0.3% hydrogen peroxide-added methanol at room temperature for 15
min to inhibit endogenous peroxidase activity. After washing with
water for 5 min, the sections were transferred to 0.01 M citrate
buffer and treated in a microwave oven for 15 min to inactivate the
antigen. After returning to room temperature, the sections were
washed with 0.01 M phosphate-buffered saline (PBS), and allowed to
stand in 2% bovine serum albumin (BSA)/PBS at room temperature for
15 min to inhibit non-specific reactions. The sections were then
incubated with monoclonal mouse anti-TS antibody and diluted
100-fold with PBS as the primary antibody at 4°C overnight.
Subsequently, the sections were washed with PBS and incubated with
peroxidase-labeled dextran 70-conjugated mouse immunoglobulin/goat
anti-polyclonal antibody and peroxidase-labeled dextran
500-conjugated anti-mouse immunoglobulin/goat anti-polyclonal
antibody as the secondary antibodies at room temperature for 30
min.
After washing with PBS again, the sections were
stained with 0.04% diaminobenzidine (DAB), nuclear stained with
hematoxylin, washed with water, dehydrated with 70, 80, 90 and 99%
ethanol, penetrated with 100% xylene, encapsulated and examined
microscopically.
Evaluation of immunostaining
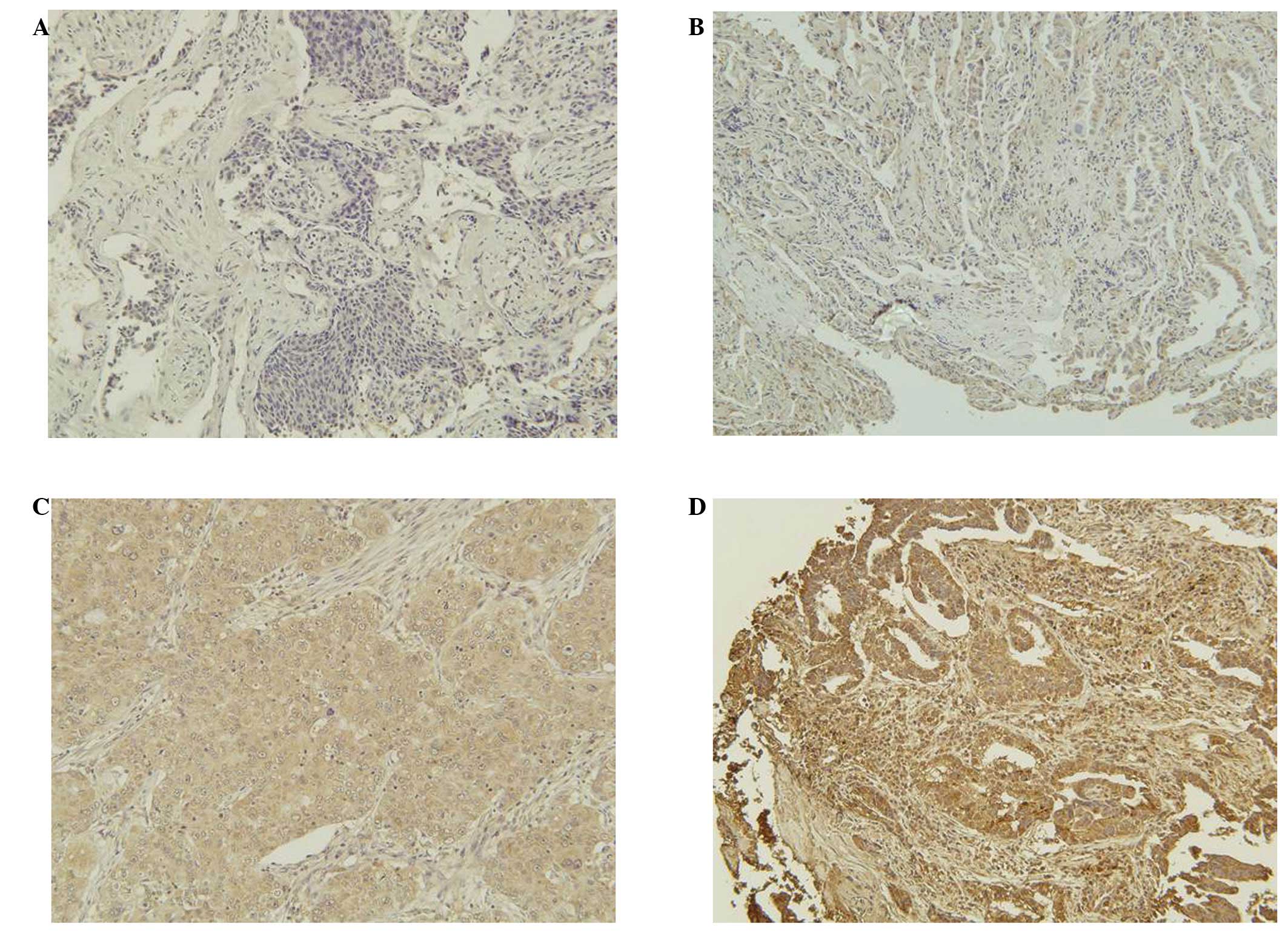
Expression of TS protein was evaluated using
H-scoring. Staining intensity was evaluated as 0 (negative,
Fig. 1A), 1 (weakly positive,
Fig. 1B), 2 (moderately positive,
Fig. 1C) or 3 (strongly positive,
Fig. 1D), and multiplied by the
proportion (%) of positive cells to calculate the H-score as
previously described (15).
Statistical analysis
To examine the effect of patient background factors,
the Mann-Whitney U test was used to compare TS protein expression
in the two groups. To evaluate the correlation between TS protein
expression and therapeutic effects, the latter were evaluated by
investigating the response rate (RR), progression-free survival
(PFS) and overall survival (OS). To compare RR between the two
groups, the Chi-square test was performed, while the log-rank test
was performed using the Kaplan-Meier method to compare PFS and OS
between the two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
TS protein expression and patient
background factors
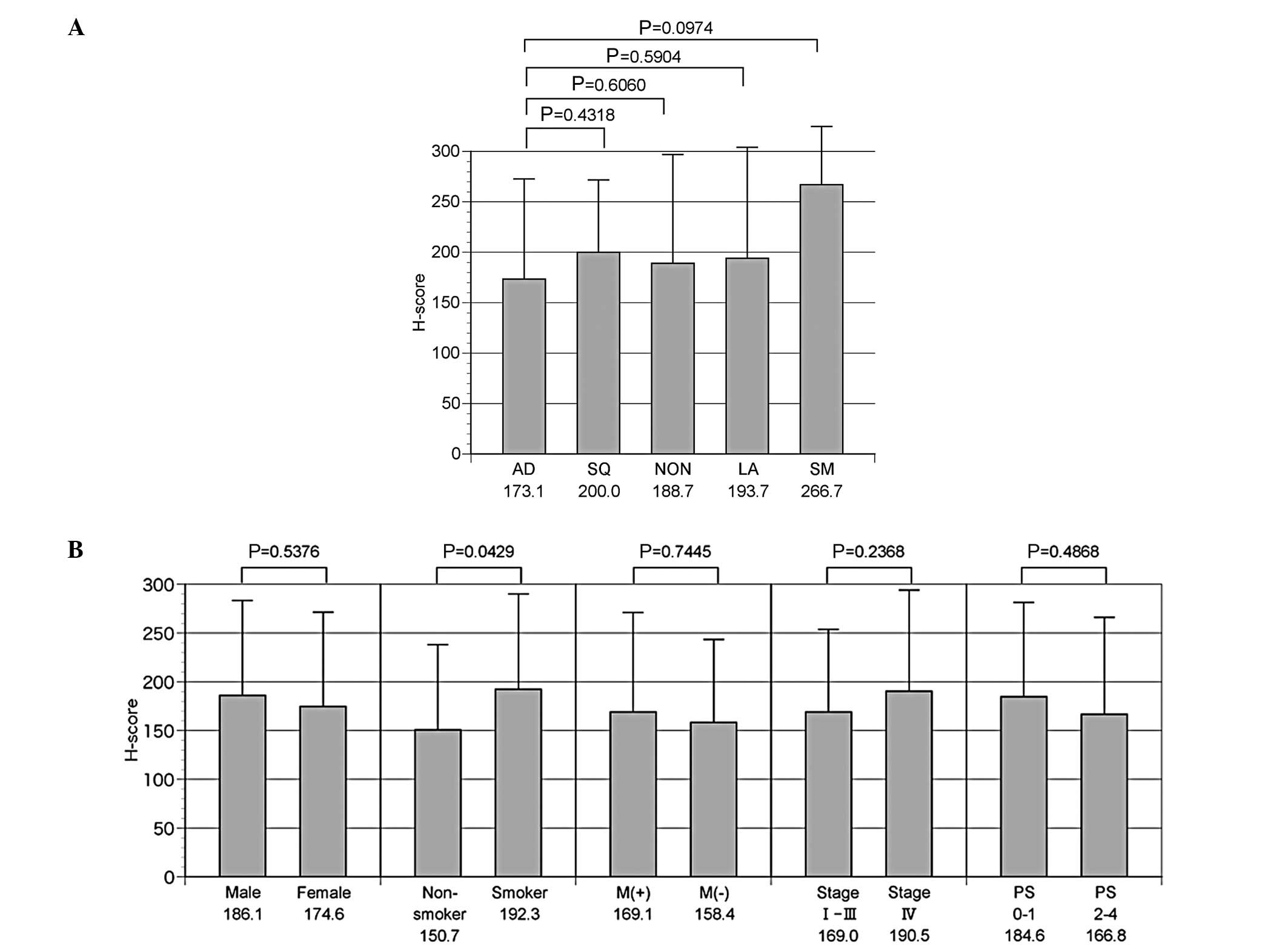
The mean H-score was 173.1±99.5 for adenocarcinoma,
200.0±71.5 for squamous cell carcinoma, 193.7±110.0 for large cell
carcinoma, 188.7± ? for non-small cell carcinoma and 266.7±57.7 for
small cell carcinoma patients. TS expression tended to be higher in
small cell carcinoma compared with adenocarcinoma (P=0.0974), while
no additional significant differences were observed among the other
types of lung cancer tissue (Fig.
2A). Regarding the additional background factors, no
significant differences in gender, presence/absence of EGFR gene
mutation or PS were observed. However, the mean H-score was
150.7±87.2 for non-smokers and 192.3±97.6 for smokers, indicating a
significantly higher expression of TS protein in smokers (P=0.0429)
(Fig. 2B).
TS protein expression and patient
survival
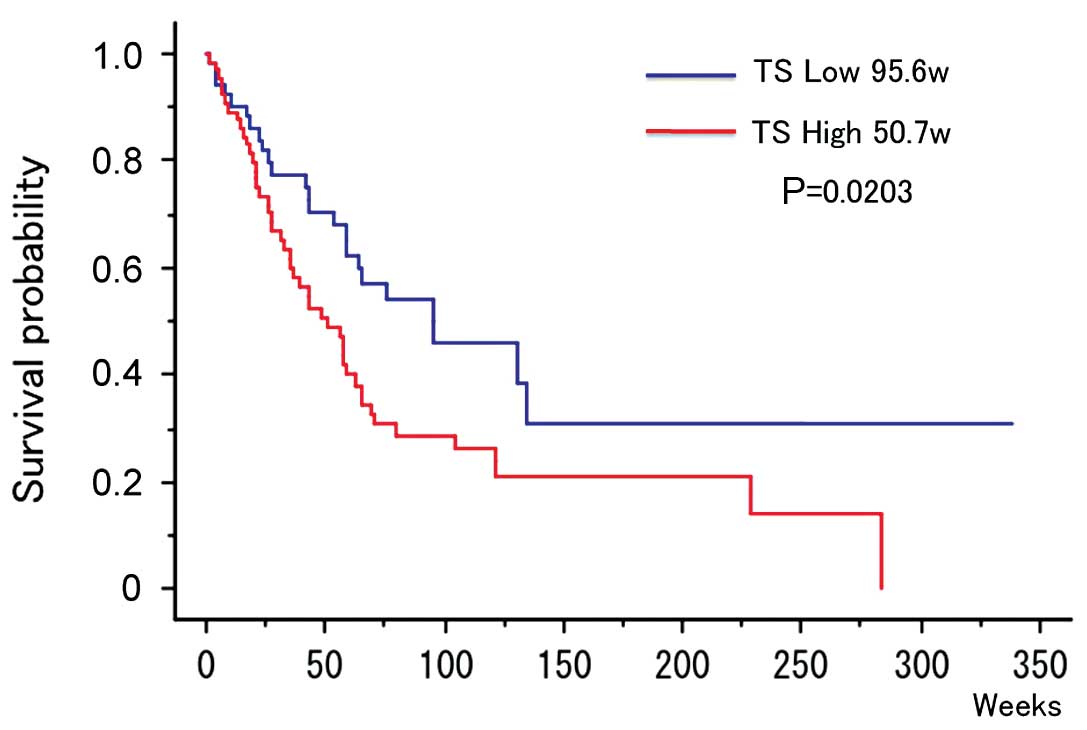
The median H-score in NSCLC patients was 200.
Patients with a higher H-score were evaluated as the high TS
protein expression group (n=63, 53.8%) and patients with a lower
H-score were evaluated as the low TS protein expression group
(n=54, 46.1%). OS (median value) was 95.6 weeks in the
low-expression and 50.7 weeks in the high-expression group,
indicating a significant prolongation of survival in the
low-expression group (P=0.0203) (Fig.
3).
TS protein expression and therapeutic
effects
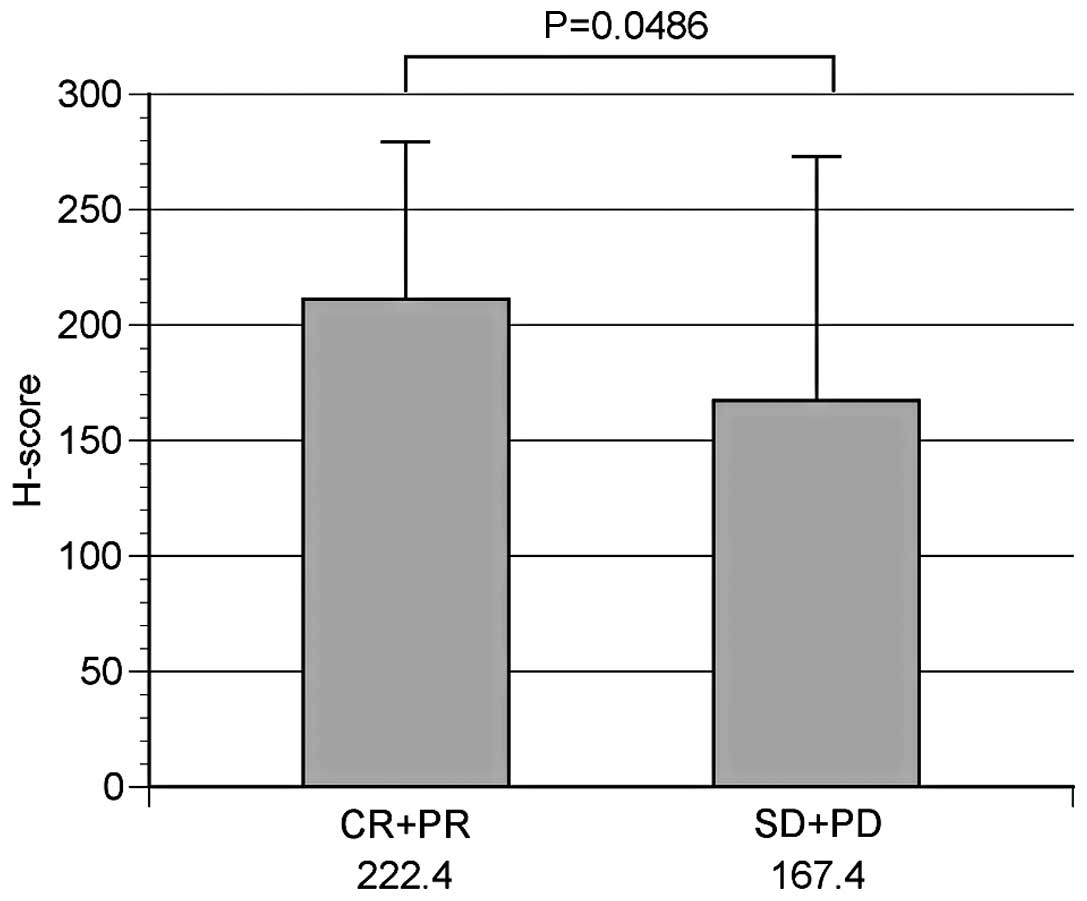
NSCLC was observed in 117 patients, 50 of whom were
administered CbPac therapy as the primary treatment. The
correlation between TS protein expression and the therapeutic
effects of CbPac therapy was, therefore, examined in these 50
patients, 31 of whom (62.0%) comprised the high TS protein
expression group and 19 (38.0%) the low TS protein expression
group. Patients whose RR was complete response (CR) (n=0) or
partial response (PR) (n=19) comprised the response group (n=19),
while those whose RR was stable disease (SD) (n=20) or progressive
disease (PD) (n=11) comprised the non-response group (n=31). The
mean H-score was 222.4±68.1 in the response and 167.4±105.6 in the
non-response groups, indicating a significantly higher TS
expression in the response group (P=0.0486) (Fig. 4).
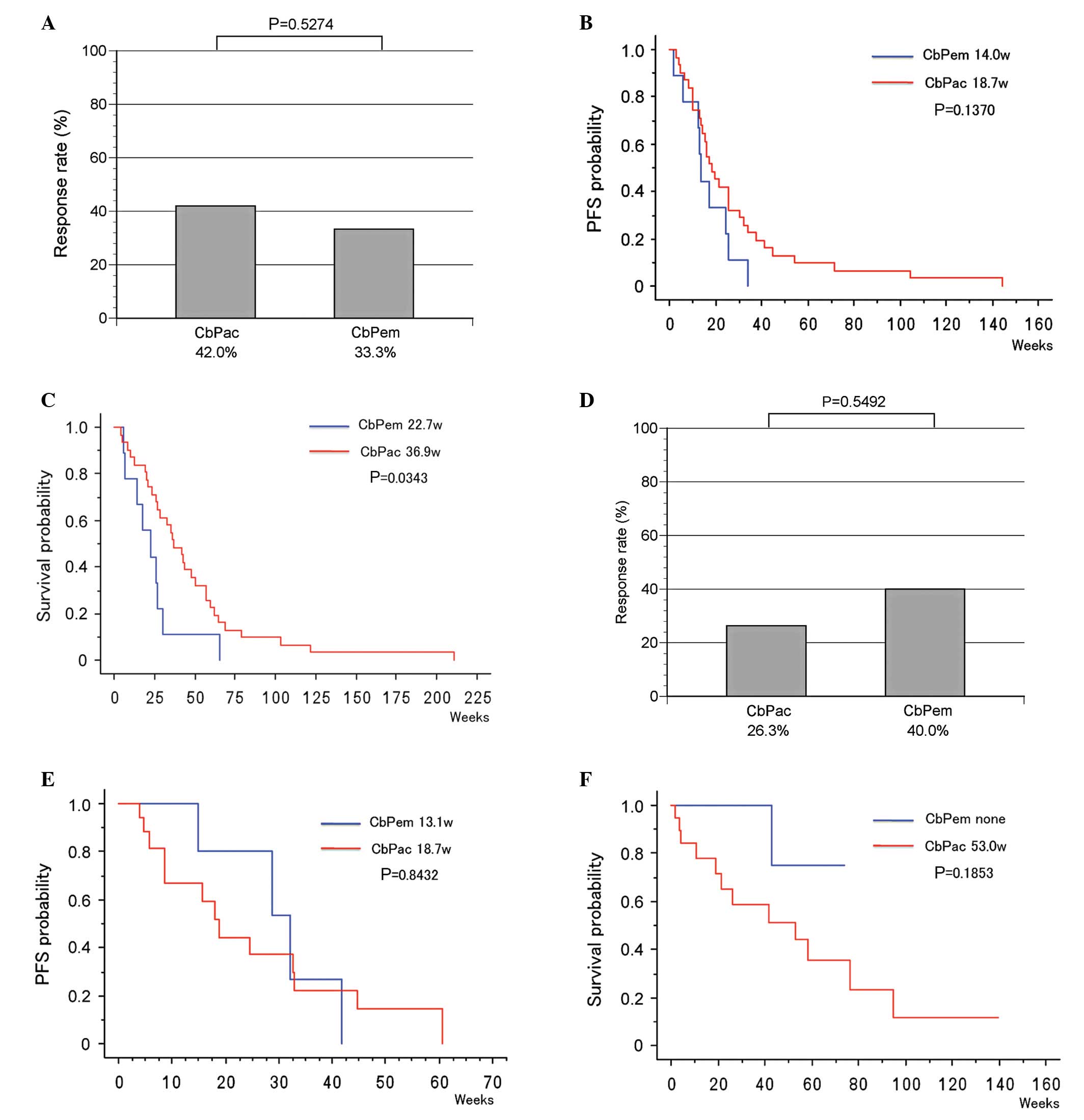
The therapeutic effects of CbPac (31 patients) and
CbPem therapies (9 patients) as the primary treatment were compared
in the high TS expression group of NSCLC patients. RR was 42.0% and
33.3% in the CbPac and CbPem therapy groups, respectively
(P=0.5274, Fig. 5A). PFS (median
value) was 18.7 and 14.0 weeks in the CbPac and CbPem therapy
groups, respectively (P=0.1370, Fig.
5B). OS (median value) was 36.9 and 22.7 weeks in the CbPac and
CbPem therapy groups, respectively, indicating a significant
prolongation of OS in the CbPac therapy group (P=0.0343, Fig. 5C).
When CbPac (19 patients) and CbPem therapies (5
patients) were compared in the low TS expression group, RR was 26.3
and 40.0% in the CbPac and CbPem therapy groups, respectively
(P=0.5492, Fig. 5D). PFS (median
value) was 18.7 and 13.1 weeks in the CbPac and CbPem therapy
groups, respectivley (P=0.8432, Fig.
5E). OS (median value) was 53.0 weeks in the CbPac therapy
group, whereas the median survival period was not achieved in the
CbPem group (P=0.1853, Fig.
5F).
Discussion
The standard treatment for advanced NSCLC is
two-drug combination therapy containing a platinum agent, which has
been shown to prolong the median survival time from 6 to 8 weeks
and improve the 1-year survival rate from 15 to 25%, as previously
demonstrated (16). One of the
recommended drugs for combination with platinum is Pac, with CbPac
therapy constituting one of the standard therapeutic methods for
the treatment of lung cancer. Chemotherapy is employed in the
treatment of advanced NSCLC. Thus, to increase its efficacy through
the selection of the most suitable drugs, predictive factors are
being investigated. One predictive factor of the efficacy of
platinum agents is the excision repair cross-complementing 1
(ERCC1) gene, a DNA repair protein (17). Class III β-tubulin is another known
prognostic factor of Pac (18).
However, whether ERCC1 and β-tubulin are also predictive factors of
the effects of CbPac therapy on advanced NSCLC remains to be
determined.
TS metabolizes 5,10-methylenetetrahydrofolate
(CH2THF) as well as deoxyuridylate-5′-monophosphate
(dUMP) by reductive methylation to deoxythymidine-5′-monophosphate
(dTMP) to produce the thymine nucleotides required for DNA
synthesis, and is involved in the biosyn-thesis of pyrimidine.
5-fluorodeoxyuridine monophosphate (FdUMP), a target enzyme of
5-fluorouracil (5-FU) and an active metabolite of 5-FU, binds to TS
and activates folic acid to promote the formation of
TS-FdUMP-CH2FH4 ternary complexes. When TS is
completely inhibited, DNA synthesis in cells is also inhibited,
resulting in antitumor effects. Accordingly, it has been reported
that when the amount of TS protein in tumor cells is high, the
antitumor effect of 5-FU is low, and when the amount is small, the
sensitivity of 5-FU is high (19).
In gastric (19), colon (11), breast (12) and pancreatic cancer (13), the correlation between TS
expression and the therapeutic effects of 5-FU and its prognosis
has been reported. Additionally, TS protein is positioned
downstream of the cell growth signal and is involved in the
proliferation of cancer cells. Therefore, the expression of TS
protein in cancer cells may influence the effects of various
anticancer drugs.
In the present study, we examined the correlation
between the expression of TS protein, tissue type, patient
background factors, prognosis and the therapeutic effects of CbPac
therapy in lung cancer. No significant difference between tissue
type and expression of TS protein was identified. However, the TS
protein expression was higher in small and squamous cell carcinoma
compared with adenocarcinoma, consistent with a previous study
(20). Regarding the correlation
between patient background factors and TS protein expression, there
was no significant difference in gender, smoking status, EGFR gene
mutation, clinical stage or PS, while TS protein expression was
higher in smokers compared with non-smokers. Since smoking has been
shown to induce mutations in genes such as p53 and KRAS (21–23),
it is likely that this increase in TS protein expression was the
result of a gene mutation induced by smoking. In addition,
significant prolongation of OS was observed in the low compared
with the high TS expression group, suggesting that TS protein
expression may affect the prognosis of lung cancer. The rate of
expression of TS protein in the primary lesion has been reported to
correlate with malignancy (24),
and is also thought to be involved in the biological malignancy of
cancer.
The investigation of the correlation between the
therapeutic effects of CbPac therapy and expression of TS protein
demonstrated a higher TS in the response compared with the
non-response group. This suggests that a higher TS expression is
closely associated with higher efficacy of CbPac therapy. Moreover,
when the effects of CbPac and CbPem therapies were compared in the
high TS expression group, RR tended to be higher and PFS longer in
the CbPac therapy group. OS was also significantly prolonged in the
CbPac therapy group. These results suggest that Pac is more
effective compared with Pem in the treatment of NSCLC with high TS
expression, suggesting a higher efficacy of CbPac therapy in the
high TS expression group, which is in contrast to the correlation
between the amount of TS protein and the antitumor effect of 5-FU.
As mentioned previously, 5-FU inhibits DNA synthesis to achieve
antitumor effects. Similarly, Pem also inhibits DNA synthesis by
inhibiting TS, a folate metabolic enzyme, to achieve antitumor
effects. However, Pac stops cell division resulting in antitumor
effects by inhibiting and stabilizing depolymerization of
microtubules in the M phase of cell division. Since TS is a
rate-limiting enzyme of DNA synthesis, a high TS expression is
thought to indicate a high rate of cell division.
When CbPac and CbPem therapies were compared in the
low TS expression group, RR was higher, while PFS and OS tended to
be prolonged in the CbPem therapy group. In the low TS expression
group, Pem was more effective compared with Pac. It has been
reported (20) that Pem inhibits
the growth of tumor cells and induces cell death mainly through the
inhibition of TS, resulting in antitumor effects. Pem also shows
high sensitivity in low TS-expressing cells. Therefore, Pem likely
has an impact on NSCLC with low TS expression.
In the present study, expression of TS protein was
examined using immunostaining methods. While quantification of mRNA
can also be used to examine protein expression, immunostaining
requires few specimens and is a simple procedure, constituting a
convenient test method in clinical practice. Currently, the
standard therapeutic method for primary treatment of NSCLC is CbPac
+ bevacizumab or cisplatin (Cis) + Pem. Therefore, prediction of
the therapeutic effects of Pac and Pem is of high clinical
relevance. Concerning the results of the present study,
determination of TS protein expression using immunostaining methods
is considered useful for the selection of Pac- or Pem-based
platinum doublet during treatment. The findings also suggest that
the selection of an effective primary treatment according to the
rate of TS protein expression in lung cancer tissues may lead to
improved prognosis, as observed in EGFR gene mutation analysis.
References
|
1.
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rosell R, Monzo M, Molina F, et al: K-ras
genotypes and prognosis in non-small-cell lung cancer. Ann Oncol.
6:S15–S20. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Takano T, Fukui T, Ohe Y, et al: EGFR
mutations predict survival benefit from gefitinib in patients with
advanced lung adenocarcinoma: a historical comparison of patients
treated before and after gefitinib approval in Japan. J Clin Oncol.
26:5589–5595. 2008. View Article : Google Scholar
|
|
5.
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ferguson PJ, Collins O, Dean NM, et al:
Antisense down-regulation of thymidylate synthase to suppress
growth and enhance cytotoxicity of 5-FUdR, 5-FU and Tomudex in HeLa
cells. Br J Pharmacol. 127:1777–1786. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Flynn J, Berg RW, Wong T, et al:
Therapeutic potential of antisense oligodeoxynucleotides to
down-regulate thymidylate synthase in mesothelioma. Mol Cancer
Ther. 5:1423–1433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lin SB, Ts’o PO, Sun SK, et al: Inhibition
of thymidylate synthase activity by antisense oligodeoxynucleotide
and possible role in thymineless treatment. Mol Pharmacol.
60:474–479. 2001.PubMed/NCBI
|
|
9.
|
Hann N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small-cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Okamoto I, Yoshioka H, Morita S, et al:
Phase III trial comparing oral S-1 plus carboplatin with paclitaxel
plus carboplatin in chemotherapy-naive patients with advanced
non-small-cell lung cancer: result of a West Japan oncology group
study. J Clin Oncol. 20:5240–5246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shirota Y, Stoehlmacher J, Brabender J, et
al: ERCC1 and thymidylate synthase mRNA level predict survival for
colorectal cancer patients receiving combination oxaliplatin and
fluorouracil chemotherapy. J Clin Oncol. 19:4298–4304. 2001.
|
|
12.
|
Nishimura R, Nagao K, Miyayama H, et al:
Thymidylate synthase levels as a therapeutic and prognostic
predictor in breast cancer. Anticancer Res. 19:5621–5626.
1999.PubMed/NCBI
|
|
13.
|
Takamura M, Nio Y, Yamasawa K, et al:
Implication of thymidylate synthase in the outcome of patients with
invasive ductal carcinoma of the pancreas and efficacy of adjuvant
chemotherapy using 5-fluorouracil or its derivatives. Anticancer
Drugs. 13:75–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zheng Z, Li X, Schell MJ, et al:
Thymidylate synthase in situ protein expression and survival in
stage I non small-cell lung cancer. Cancer. 112:2765–2773. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Chen CY, Chang YL, Shin JY, et al:
Thymidylate synthase and dihydrofolate reductase expression in
non-small cell lung carcinoma: the association with treatment
efficacy of pemetrexed. Lung Cancer. 74:132–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Non-small Cell Lung Cancer Collaborative
Group: Chemotherapy in non-small cell lung cancer: a meta-analysis
using updated date on individual patients from 52 randomized
clinical trials. BMJ. 311:899–909. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Olaussen KA, Dunant A, Fouret P, et al
IALT Bio Investigators: DNA repair by ERCC1 in non-small cell lung
cancer and cisplatin-based adjuvant chemotherapy. N Eng J Med.
355:983–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yin S, Bhattacharya R and Cabral F: Human
mutations that confer paclitaxel resistance. Mol Cancer Ther.
9:327–335. 2010. View Article : Google Scholar
|
|
19.
|
Lenz HJ, Leichman CG, Danenberg KD, et al:
Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a
predictor for primary tumor response and overall survival. J Clin
Oncol. 14:176–182. 1996.PubMed/NCBI
|
|
20.
|
Ceppi P, Volante M, Saviozzi S, et al:
Squamous cell carcinoma of the lung compared with other histotypes
shows higher messenger RNA and protein levels for thymidylate
synthase. Cancer. 107:1589–1596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Suzuki H, Takahashi T, Kuroishi T, et al:
p53 mutations in non-small cell lung cancer in Japan: association
between mutations and smoking. Cancer Res. 52:734–736.
1992.PubMed/NCBI
|
|
22.
|
Ahrendt SA, Decker PA, Alawi EA, et al:
Cigarette smoking is strongly associated with mutation of the K-ras
gene in patients with primary adenocarcinoma of the lung. Cancer.
92:1525–1530. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Mitsudomi T, Oyama T, Nishida K, et al:
Loss of heterozygosity at 3p in non-small cell lung cancer and its
prognostic implication. Clin Cancer Res. 2:1185–1189.
1996.PubMed/NCBI
|
|
24.
|
Johnston PG, Fisher ER, Rocktte HE, et al:
The role of thymidylate synthase expression in prognosis and
outcome of adjuvant chemotherapy in patients with rectal cancer. J
Clin Oncol. 12:2640–2647. 1994.PubMed/NCBI
|