Introduction
Epigenetic alterations are important in
carcinogenesis, with methylation of the promoter part of tumor
suppressor genes constituting one of the important alterations.
Although this mechanism can be detected by methylation-specific
polymerase chain reaction (PCR), it is difficult to perform such an
investigation in pathological specimens. In addition, observation
of methylation is complicated as compared with pathological
findings. The representative protein in the DNA methyltransferase
(DNMT) family of proteins, which has important roles in the
methylation of DNA, is DNA methyltransferase 1 (DNMT1) (1). Methylation of a general tumor can be
examined by detecting DNMT1 with immunostaining methods, a
procedure that is easily performed in a general hospital and it has
been reported that overexpression of DNMT1 protein is correlated
with the methylation of tumor suppressor genes (2).
An endometrial carcinoma is representative of
hormonal-dependent tumors, with endometrioid adenocarcinoma, which
arises from endometrial hyperplasia, constituting the most
frequently encountered type. Methylation is known to occur at a
high frequency in this type of adenocarcinoma. Tumor development
has been reported to be correlated with the frequency of
methylation (2), although the
mechanism has yet to be investigated in detail. Using
immunostaining methods, we aimed to investigate the expression of
hMLH1 and E-cadherin, which have been reported to be attenuated
with tumor development in the endometrium (3), and to determine their correlation
with the overexpression of DNMT1. In addition, an in situ
hybridization (ISH) method was used to determine whether
overexpression of DNMT1 is caused by the expression of microRNA
(mRNA) and/or gene amplification of DNMT1.
Materials and methods
Subjects
We examined endometrial biopsy tissues resected from
patients examined at the Tsuchiura Kyodo General Hospital (Ibaraki,
Japan) from 2001 to 2008. Samples were obtained from 8 cases of
normal tissue, 10 cases of cystic or adenomatous hyperplasia, 11
cases of atypical hyperplasia and 38 cases of endometrioid
adenocarcinoma. This study was approved by the ethics committee of
Tsuchiura Kyodo General Hospital. Informed consent was obtained
from all subjects. The tissues were embedded in formalin-fixed
paraffin sections, with a formalin fixation time of <48 h. The
age of the examined patients ranged from 19 to 88 years.
Histological diagnosis was performed by two pathologists based on
the Japanese Classification of Endometrial Carcinoma (4).
Immunostaining
Deparaffinization was performed using xylene, then
the immunostaining process was performed as described. Sections
were initially re-hydrated using an alcohol series. For hMLH1 and
E-cadherin, heat treatment in a microwave oven was performed in
citric acid buffer at pH 6.0 for 15 min, while for DNMT1 heat
treatment was performed with an autoclave in target retrieval
solution (Nichirei Bioscience, Tokyo, Japan) at pH 9.0 for 15 min.
The sections were air-cooled for 20 min, then
H2O2 treatment was performed for 10 min to
inactivate endogenous peroxidase. Anti-hMLH1 mouse monoclonal
(ES05, dilution, 1:100; Leica Microsystems, Newcastle Upon Tyne,
UK), anti-E-cadherin rabbit polyclonal (dilution, 1:200; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and anti-DNMT1 mouse
monoclonal (60B1220.1, dilution, 1:300; Acris Antibodies GmbH,
Herford, Germany) antibodies were added to the sections in a
moisture chamber and incubated at room temperature for 3 h. After
washing in phosphate-buffered saline (PBS) for 30 min, a polymer
method was performed at room temperature with the NovoLink™ polymer
HRP kit (Leica Microsystems, Tokyo, Japan). The post-primary
antibody and horseradish peroxidase (HRP)-conjugated polymer in the
kit were incubated for 30 min each, and visualization was carried
out with 3,3′-diaminobenzidine. Counterstaining with hematoxylin,
as well as dehydration and cover slipping were then performed.
mRNA in situ hybridization
DNMT1 mRNA expression in 8 normal cases, 10 cases
with hyperplasia, 11 cases with atypical hyperplasia and 19 cases
with carcinoma was investigated. The oligonucleotide probe sequence
was 5′-GTTGCAGTCCTCTGTGAACACTGTGG-3′, as previously reported by
Mizuno et al(5). ISH was
performed according to the manufacturer’s instuctions, with a
modest modification of the digoxigenin (DIG)-tailed probe. Sections
were treated for 10 min each with pepsin, HCl, triethanolamine, and
acetic acid and then incubated with a DNMT1-specific DIG-tailed
probe at 42°C overnight. DNMT1 mRNA expression was then detected
using the GenPoint kit (Dako, Glostrup, Denmark), with an anti-DIG
antibody (dilution, 1:2,500; Roche Diagnostics, Tokyo, Japan) used
for the detection of DIG instead of the streptavidin included in
the kit.
Chromogenic in situ hybridization
The DNA probe for chromogenic in situ
hybridization (CISH) was produced using a Probe Synthesis kit
(Roche Diagnostics). The primer used to detect DNMT1 was designed
using free software primer3, with reference to information provided
by the National Center for Biotechnology Information (NCBI). The
reference sequence was NG_028016.1. The forward and reverse primer
sequences were GACCTCCTCCTCTGTTGCAG and AGACCAGGGGTCACACAAAG,
respectively. The PCR product (348 bp) was used as the CISH probe
and labeled with DIG by the kit. CISH was performed using this
probe, and detection was carried out using the GenPoint kit (Dako)
in the same manner as mRNA detection. CISH was performed in 48
cases in the same manner as mRNA ISH.
Assessments and statistical analysis
Immunocytochemical staining of DNMT1 was considered
positive when ≥20% of the tumor cells expressed the protein in the
nucleus (6). The color development
of the cytoplasm was not taken into consideration for this
determination. hMLH1 and E-cadherin were regarded as negative when
≥10% of the tumor cells in the membrane were attenuated. As for
ISH, cases in which the cytoplasm in ≥10% of the tumor cells was
stained were considered positive. Intra-nuclear signals of the
DNMT1 gene in at least 20 nuclei per case were quantified and the
average was calculated. Statistical analyses for associations were
performed using the Student’s t-test or the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Immunostaining
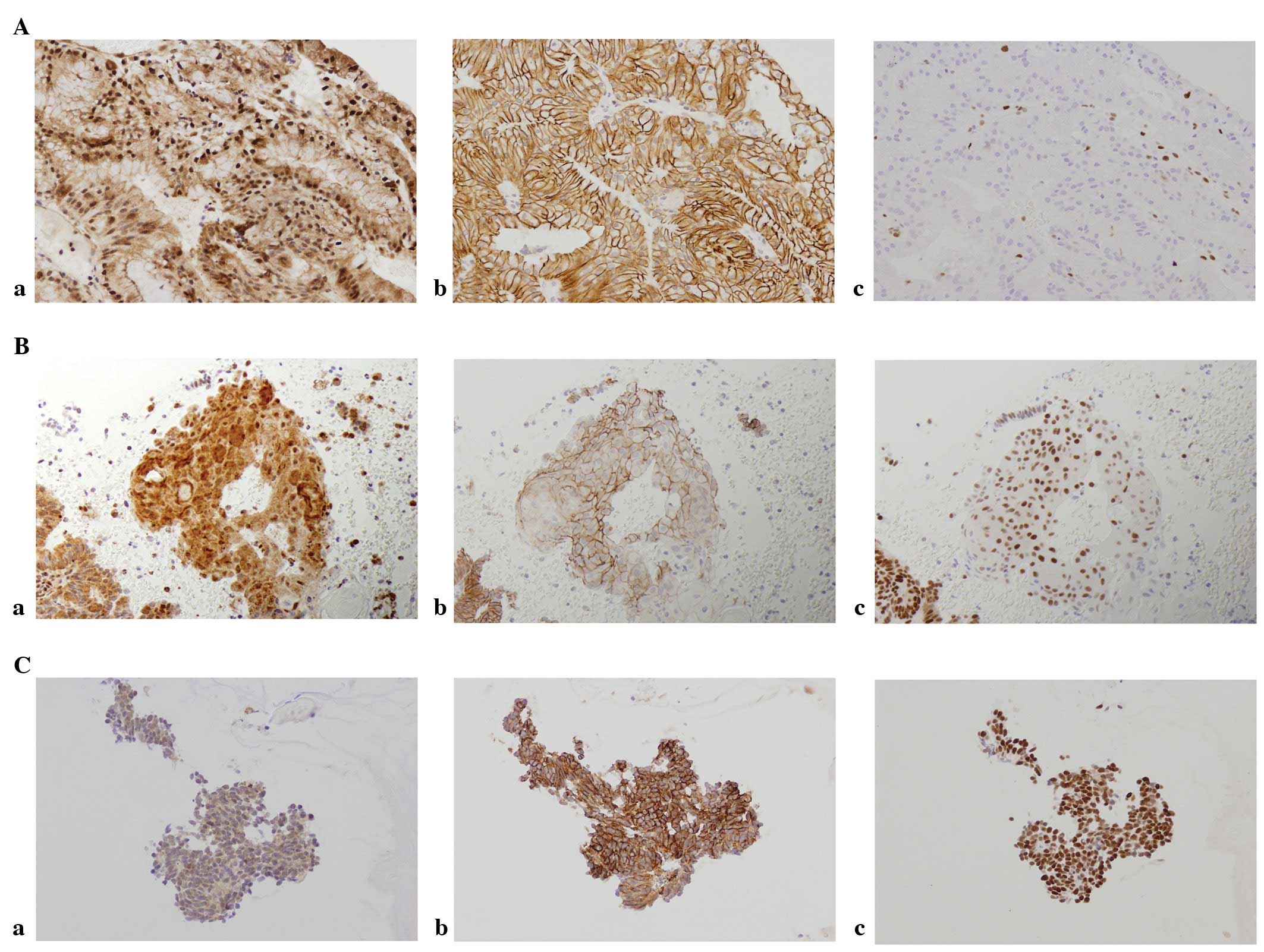
Immunostaining results showed that DNMT1 and hMLH1
proteins were expressed in the nucleus, whereas E-cadherin was
expressed in the membrane (Fig.
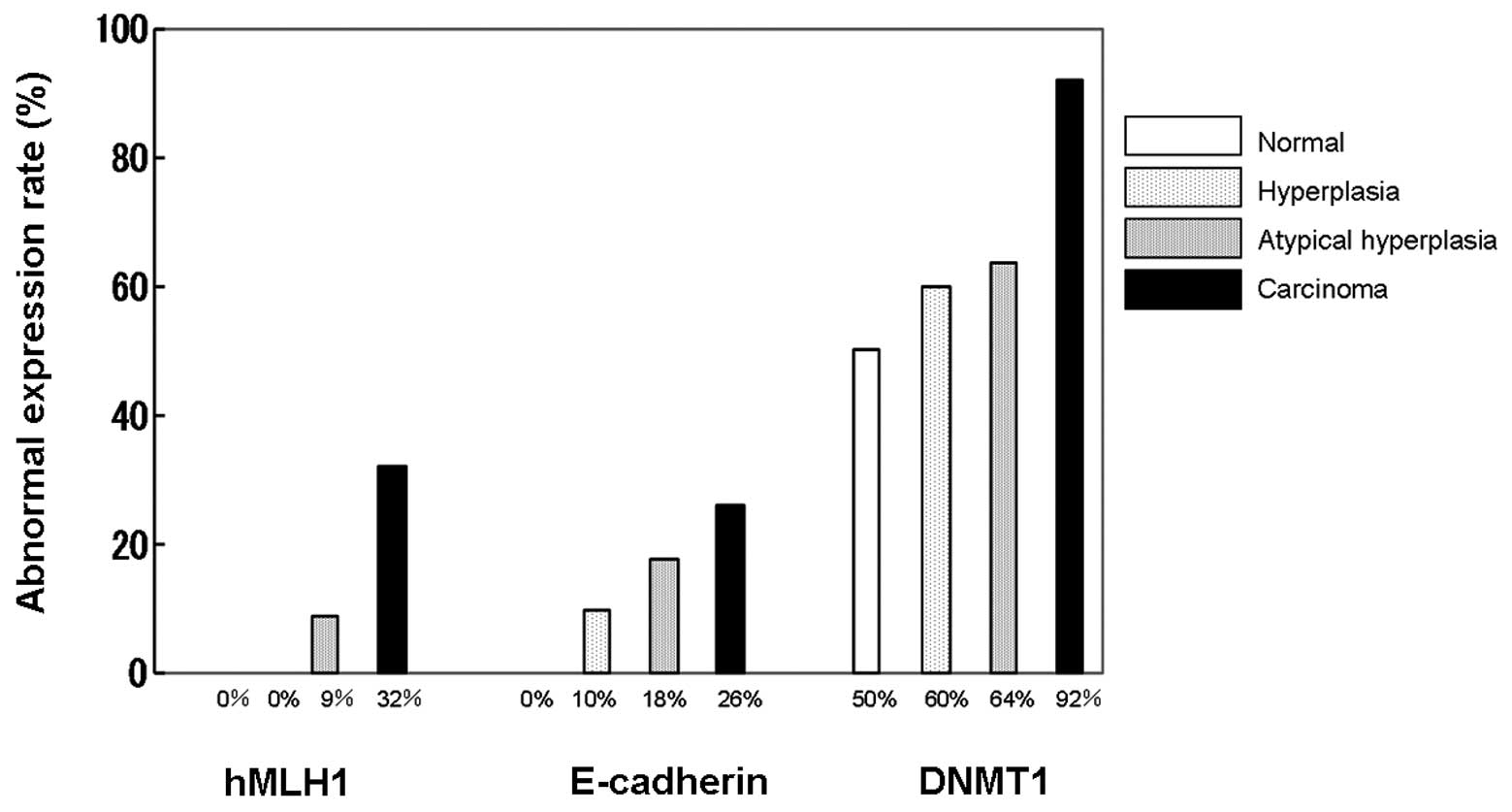
1). The expression of DMNT1 was observed even in normal tissue,
with 50% of normal tissues evaluated as positive. Positive cases
increased with tumor development and 92% of the cases in the
carcinoma group demonstrated positivity. Expression of hMLH1 in the
nucleus was observed in all the cases of normal tissue, while
attenuated cases increased with tumor development and 32% of the
cases in the carcinoma group were negative. Similar findings were
obtained for E-cadherin, with 26% of the cases in the carcinoma
group evaluated as negative (Fig.
2).
Tumor development was significantly associated with
the expression of DNMT1 and hMLH1 (Table I). In addition, the expression of
DNMT1 was significantly correlated with the expression of hMLH1 and
E-cadherin (Table II). However, no
significant correlation was observed with the carcinoma group
(Table III). In addition, there
were no cases negative for hMLH1 or E-cadherin among the
DNMT1-negative cases, and none of the cases lacked hMLH1 and
E-cadherin expression.
 | Table ICorrelation between protein expression
levels and tumor development. |
Table I
Correlation between protein expression
levels and tumor development.
| Protein
expression | Cancerous (n) | Non-cancerous
(n) | P-value |
|---|
| hMLH1 | | | |
| Positive | 28 | 26 | 0.0048 |
| Negative | 1 | 12 | |
| E-cadherin | | | |
| Positive | 26 | 28 | 0.13 |
| Negative | 3 | 10 | |
| DNMT1 | | | |
| Positive | 17 | 35 | 0.0023 |
| Negative | 12 | 3 | |
 | Table IICorrelation among the expression
levels of the three examined proteins. |
Table II
Correlation among the expression
levels of the three examined proteins.
| Protein
expression | hMLH1
|
| Positive | Negative | P-value |
|
| E-cadherin | | | |
| Positive | 41 | 13 | 0.058 |
| Negative | 13 | 0 | |
|
| hMLH1
|
| Positive | Negative | P-value |
|
| DNMT1 | | | |
| Positive | 39 | 13 | 0.031 |
| Negative | 15 | 0 | |
|
| E-cadherin
|
| Positive | Negative | P-value |
|
| DNMT1 | | | |
| Positive | 39 | 13 | 0.031 |
| Negative | 15 | 0 | |
 | Table IIICorrelation among the expression
levels of the three examined proteins (carcinoma cases only). |
Table III
Correlation among the expression
levels of the three examined proteins (carcinoma cases only).
| Protein
expression | hMLH1
|
| Positive | Negative | P-value |
|
| E-cadherin | | | |
| Positive | 16 | 12 | 0.28 |
| Negative | 10 | 0 | |
|
| hMLH1
|
| Positive | Negative | P-value |
|
| DNMT1 | | | |
| Positive | 23 | 12 | 0.22 |
| Negative | 3 | 0 | |
|
| DNMT1
|
| Positive | Negative | P-value |
|
| E-cadherin | | | |
| Positive | 16 | 12 | 0.28 |
| Negative | 10 | 0 | |
mRNA in situ hybridization
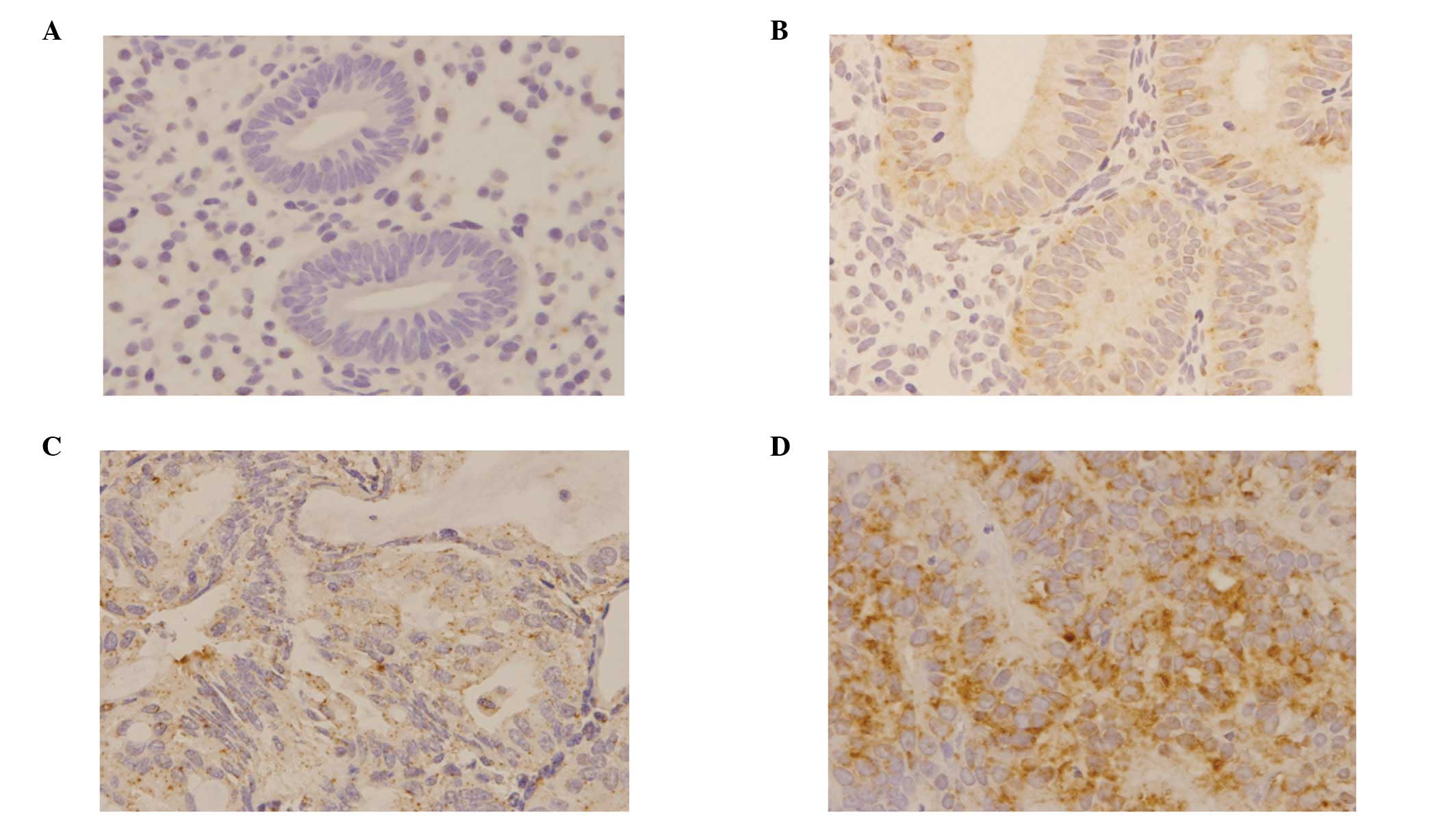
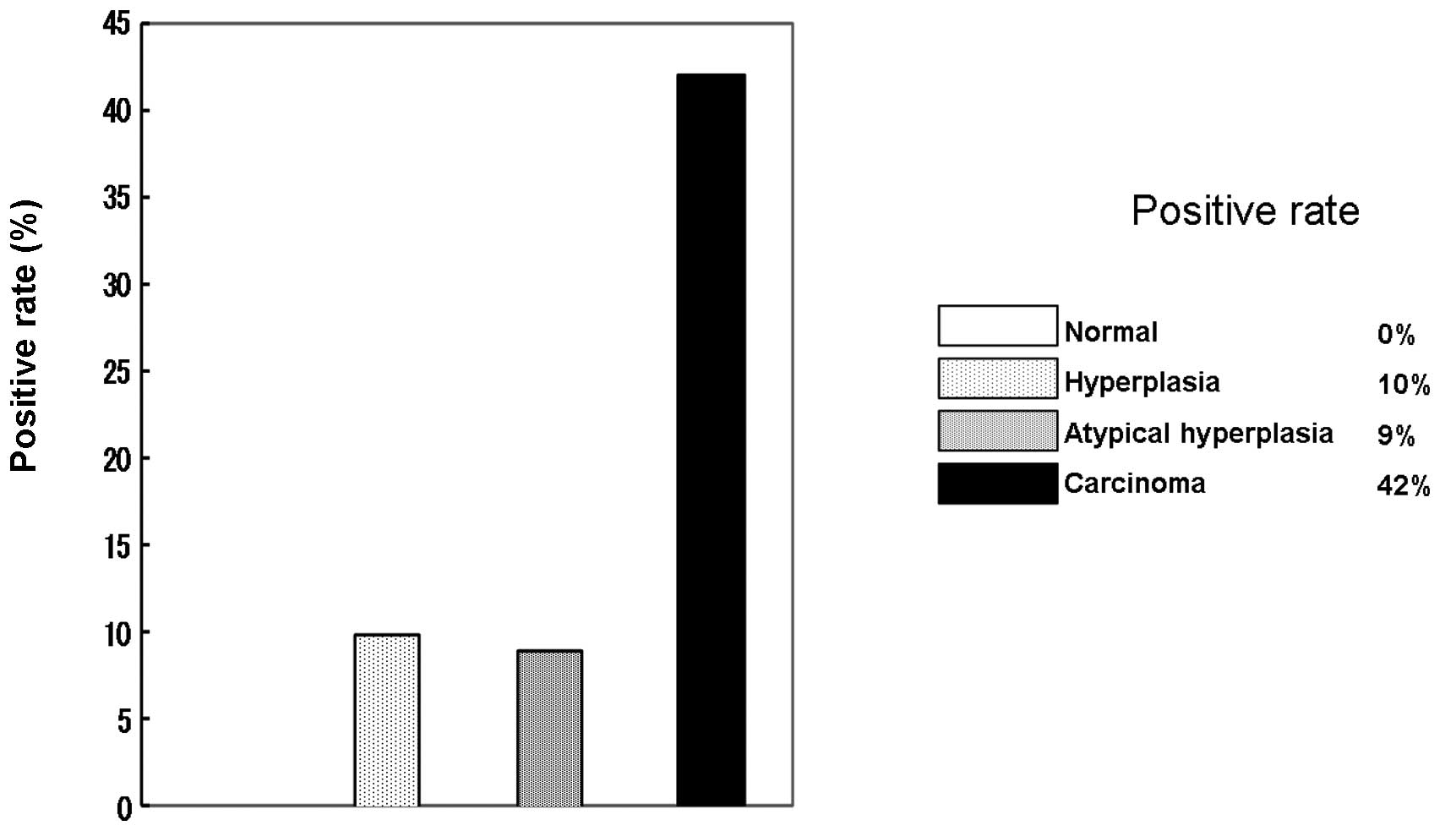
The expression of DNMT1 mRNA was examined using an
ISH method (Fig. 3). DNMT1 mRNA
was completely absent in all the normal cases, while only a few
cases in the hyperplasia and atypical hyperplasia groups were
positive. However, a high expression level was observed in
carcinoma cases, 42% of which were positive (Fig. 4). In addition, a significant
correlation between DNMT1 mRNA expression and attenuation of hMLH1
protein was observed (Table
IV).
 | Table IVCorrelation between tumor development,
protein expression and DNMT1 mRNA. |
Table IV
Correlation between tumor development,
protein expression and DNMT1 mRNA.
| Protein
expression | DNMT1 mRNA expression
|
|---|
| Positive | Negative | P-value |
|---|
| Non-cancerous | 2 | 27 | 0.0082 |
| Cancerous | 8 | 11 | |
| hMLH1 | | | |
| Positive | 5 | 34 | 0.012 |
| Negative | 5 | 4 | |
| DNMT1 | | | |
| Positive | 8 | 26 | 0.7 |
| Negative | 2 | 12 | |
| E-cadherin | | | |
| Positive | 10 | 31 | 0.32 |
| Negative | 0 | 7 | |
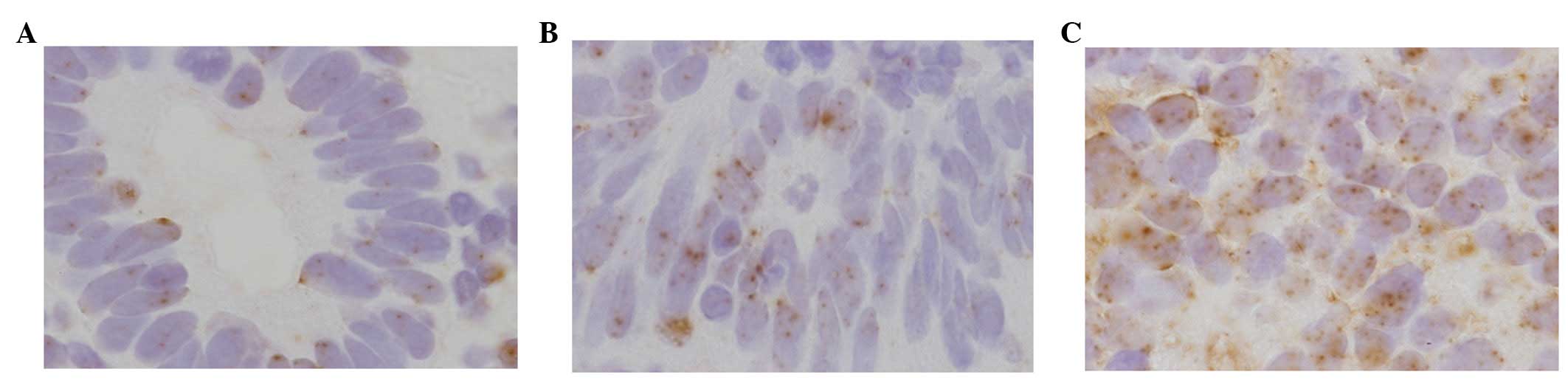
Chromogenic DNA in situ
hybridization
DNMT1 genetic amplification using the CISH method
was also performed (Fig. 5) in
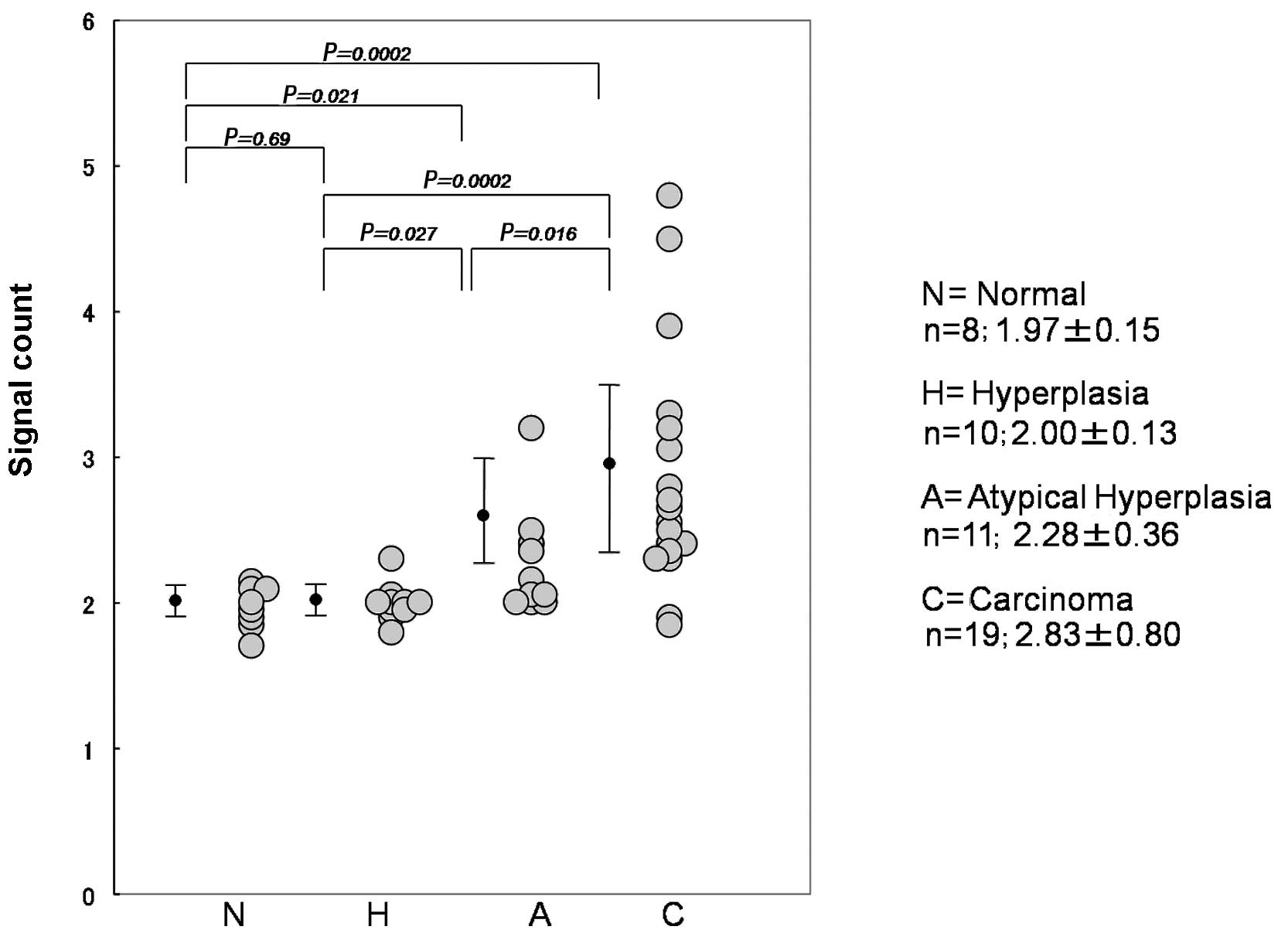
each case, showing that the average number of signals increased
with tumor development. The average number in normal, hyperplasia,
atypical hyperplasia and carcinoma cases was 1.97, 2.00, 2.28 and
2.83, respectively (Fig. 6).
Furthermore, DNMT1 genetic amplification was found to be correlated
with the expression of DNMT1 protein (Table V).
 | Table VCorrelation between gene
amplification of DNMT1 and protein expression levels. |
Table V
Correlation between gene
amplification of DNMT1 and protein expression levels.
| Protein
expression | N | Average of
signals | P-value |
|---|
| hMLH1 | | | |
| Positive | 39 | 2.34±0.66 | 0.37 |
| Negative | 9 | 2.57±0.64 | |
| E-cadherin | | | |
| Positive | 41 | 2.34±0.60 | 0.30 |
| Negative | 7 | 2.63±0.92 | |
| DNMT1 | | | |
| Positive | 34 | 2.47±0.74 | 0.041 |
| Negative | 14 | 2.17±0.27 | |
| mRNA | | | |
| Positive | 10 | 2.81±0.91 | 0.10 |
| Negative | 38 | 2.28±0.53 | |
Discussion
In a previous study, we investigated the expression
of DNMT1, an important protein in the maintenance of methylation,
in relation to the expression of hMLH1 and E-cadherin, which is
often observed in endometrioid carcinomas (6). In the present study, mRNA expression
and gene amplification of DNMT1 were investigated, and results
showed that they were increased with tumor development. These
results suggest that DNMT1 mRNA expression and/or genetic
amplification may be associated with excessive methylation in
endometrial carcinomas.
DNMT1 was initially cloned in 1988 (2). Methylation is regarded as
indispensable for life in animals, as mice die following DNMT1 gene
removal (7). DNMT1 is developed
during mitosis and methylation of the same locus is maintained
throughout mitosis by DNMT1. In cases of bladder cancer, it has
been reported that DNMT1 is developed in precancerous tissue around
the carcinoma (8). In addition,
the expression of DNMT1 has also been reported in chronic hepatitis
(9,10) and ulcerative colitis (11). In the present study, DNMT1
expression was observed in half of the normal tissue samples, thus
aberrant methylation may occur at a high frequency in the
endometrium.
DNMT1 overexpression has been shown to be correlated
with tumor development in a variety of types of malignant tumors,
while DNMT1 expression in hepatoma (12) and pancreatic carcinoma (13) has been reported to be useful as an
indicator of poor prognosis. In this study, the expression of DNMT1
was observed in most cases of endometrioid adenocarcinoma.
Endometrial carcinomas are known to frequently occur in patients
with Lynch syndrome, which is characterized by gene mutations such
as hMLH1. None of the patients included in the study were confirmed
to have Lynch syndrome, and most were thought to have sporadic
cancer (14). Since DNMT1 was
detected in most of the carcinoma cases included in the study, it
is suggested that most of the tumors were developed by accumulation
of methylation, unlike other types of tumors.
The attenuation of hMLH1 and E-cadherin in
endometrial tissues was also investigated, and a correlation with
DNMT1 expression was identified. Previous studies have shown that
methylation of hMLH1 and E-cadherin occur at high frequency in
endometrial cancer (3,15). Therefore, one of the causes of the
attenuation of hMLH1 and E-cadherin may be methylation of the
promoter part by DNMT1. We re-examined this correlation in the
carcinoma group, however, the correlation was not significant,
possibly due to the low number of cases examined.
DNMT1 mRNA expression was investigated using the ISH
method. The expression was low in normal, hyperplasia and atypical
hyperplasia tissues, while it was significantly high in the
carcinoma cases in which DNMT1 mRNA was thought to have developed
in a constant manner. mRNA expression was also significantly
associated with the attenuation of hMLH1, albeit not with the
attenuation of E-cadherin. Regarding the cause of attenuation of
E-cadherin, various reasons were considered. For example, Snail,
which functions as an inhibiting factor, has been considered to
have an effect in addition to methylation (16). However, we consider that most of
the attenuation of hMLH1 is caused by methylation of the promoter
part, thus, indicating a significant association.
Furthermore, the condition of DNMT1 gene
amplification in endometrial tissues was investigated using the
CISH method, which has been rarely reported. It was observed that
the DNMT1 gene was slightly amplified in relation to tumor
development. In carcinoma cases, 3 or 4 signals were typically
observed in each nucleus, while there were no cases with extensive
gene amplification up to ∼10 to 20-fold, as often observed in cases
of breast cancer. We considered that the minor amplification of DNA
might accompany carcinogenesis. Since DNMT1 expression was found to
be associated with DNMT1 gene amplification, it is possible that it
is strengthened by gene amplification.
It was previously reported that DNMT1 expression
does not only occur with proliferative activity (8). In the present study, we confirmed
that there was no significant correlation between the expression of
DNMT1 and Ki-67, the latter of which is utilized as a marker of
proliferative activity (data not shown). As yet, aberration of
proliferative activity in cancer has not been elucidated in detail.
However, activation of DNMT1 is expected to be induced by a
mechanism that is different from cell growth.
In conclusion, our findings demonstrate that
methylation is associated with carcinogenesis in the endometrium.
Although DNMT1 protein is expressed in normal endometrial tissue, a
constant expression of DNMT1 mRNA contributes to cancerization.
DNMT1 gene amplification is associated with DNMT1 protein
expression, although that is only one of the causes of DNMT1
activation in tumor development, with genetic activation in the
upper streams of DNMT1 thought to be another. Additional studies
are required to investigate this correlation.
References
|
1.
|
Bestor T, Laudano A, Mattaliano R and
Ingram V: Cloning and sequencing of a cDNA encoding DNA
methyltransferase of mouse cells. The carboxyl-terminal domain of
the mammalian enzymes is related to bacterial restriction
methyltransferases. J Mol Biol. 203:971–983. 1988. View Article : Google Scholar
|
|
2.
|
Etoh T, Kanai Y, Ushijima S, Nakagawa T,
Nakanishi Y, Sasako M, Kitano S and Hirohashi S: Increased DNA
methyltransferase 1 (DNMT1) protein expression correlates
significantly with poorer tumor differentiation and frequent DNA
hypermethylation of multiple CpG islands in gastric cancers. Am J
Pathol. 164:689–699. 2004. View Article : Google Scholar
|
|
3.
|
Banno K, Yanokura M, Susumu N, Kawaguchi
M, Hirao N, Hirasawa A, Tsukazaki K and Aoki D: Relationship of the
aberrant DNA hypermethylation of cancer-related genes with
carcinogenesis of endometrial cancer. Oncol Rep. 16:1189–1196.
2006.PubMed/NCBI
|
|
4.
|
Japan Society of Obstetrics and
Gynecology: The General Rules for Clinical and Pathological
Management of Uterine Corpus Cancer. 3rd edition. Kinbara Shuppan;
Tokyo: 2012
|
|
5.
|
Mizuno S, Chijiwa T, Okamura T, Akashi K,
Fukumaki Y, Niho Y and Sasaki H: Expression of DNA
methyltransferases DNMT1, 3A, and 3B in normal hematopoiesis and in
acute and chronic myelogenous leukemia. Blood. 97:1172–1179. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liao X, Siu MK, Chan KY, Wong ES, Ngan HY,
Chan QK, Li AS, Khoo US and Cheung AN: Hypermethylation of RAS
effector related genes and DNA methyltransferase 1 expression in
endometrial carcinogenesis. Int J Cancer. 123:296–302. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Li E, Bestor TH and Jaenisch R: Targeted
mutation of the DNA methyltransferase gene results in embryonic
lethality. Cell. 69:915–926. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Nakagawa T, Kanai Y, Saito Y, Kitamura T,
Kakizoe T and Hirohashi S: Increased DNA methyltransferase 1
protein expression in human transitional cell carcinoma of the
bladder. J Urol. 170:2463–2466. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kondo Y, Kanai Y, Sakamoto M, Mizokami M,
Ueda R and Hirohashi S: Genetic instability and aberrant DNA
methylation in chronic hepatitis and cirrhosis - A comprehensive
study of loss of heterozygosity and microsatellite instability at
39 loci and DNA hypermethylation on 8 CpG islands in microdissected
specimens from patients with hepatocellular carcinoma. Hepatology.
32:970–979. 2000.
|
|
10.
|
Saito Y, Kanai Y, Sakamoto M, Saito H,
Ishii H and Hirohashi S: Expression of mRNA for DNA
methyltransferases and methyl-CpG-binding proteins and DNA
methylation status on CpG islands and pericentromeric satellite
regions during human hepatocarcinogenesis. Hepatology. 33:561–568.
2001. View Article : Google Scholar
|
|
11.
|
Fujii S, Katake Y and Tanaka H: Increased
expression of DNA methyltransferase-1 in non-neoplastic epithelium
helps predict colorectal neoplasia risk in ulcerative colitis.
Digestion. 82:179–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Saito Y, Kanai Y, Nakagawa T, Sakamoto M,
Saito H, Ishii H and Hirohashi S: Increased protein expression of
DNA methyltransferase (DNMT) 1 is significantly correlated with the
malignant potential and poor prognosis of human hepatocellular
carcinomas. Int J Cancer. 105:527–532. 2003. View Article : Google Scholar
|
|
13.
|
Peng DF, Kanai Y, Sawada M, Ushijima S,
Hiraoka N, Kosuge T and Hirohashi S: Increased DNA
methyltransferase 1 (DNMT1) protein expression in precancerous
conditions and ductal carcinomas of the pancreas. Cancer Sci.
96:403–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hampel H, Frankel WL, Martin E, Arnold M,
Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J,
Panescu J, Fix D, Lockman J, Comeras I and de la Chapelle A:
Screening for Lynch syndrome (hereditary nonpolyposis colorectal
cancer) among endometrial cancer patients. Cancer Res.
66:7810–7817. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Saito T, Nishimura M, Yamasaki H and Kudo
R: Hypermethylation in promoter region of E-cadherin gene is
associated with tumor dedifferention and myometrial invasion in
endometrial carcinoma. Cancer. 97:1002–1009. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Blechschmidt K, Kremmer E, Hollweck R,
Mylonas I, Höfler H, Kremer M and Becker KF: The E-cadherin
repressor snail plays a role in tumor progression of endometrioid
adenocarcinomas. Diagn Mol Pathol. 16:222–228. 2007. View Article : Google Scholar : PubMed/NCBI
|