Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of cancer worldwide (1). However, only a minority of HCC
patients benefit from curative therapies, such as surgical
resection, liver transplantation, or percutaneous treatment, since
the majority of HCCs are diagnosed at intermediate or advanced
stages (2). For patients with
unresectable HCC, the goal of palliative treatment is to control
symptoms and prolong survival. Transarterial chemoembolization
(TACE) plays an important role in palliative treatment, although
the effect of TACE on unresectable disease remains controversial
(3). Several previous randomized
trials suggested that TACE exerts little effect on survival
(4–6). However, two more recent randomized
trials reported that TACE improved the survival of patients with
unresectable HCC (7,8).
The above mentioned data on the association of TACE
with survival in unresectable HCC are mainly obtained from Western
populations. However, Western populations differ from the Chinese
population in certain aspects, such as ethnicity, etiology of HCC
and use of the TACE regimen (1).
Thus far, only one randomized controlled trial investigating TACE
for unresectable HCC was conducted in Hong Kong, China and reported
that TACE improved survival (8).
However, this trial was limited by its limited sample size,
recruiting only 80 patients.
Available data regarding the effect of TACE on
unresectable HCC are currently limited; therefore, it has not yet
been determined whether TACE is beneficial to patients with
unresectable HCC in the Chinese mainland, although the incidence of
HCC in this area accounts for >50% of the total HCC cases
worldwide (1). Several patients
receive symptomatic treatment due to the high cost or
unavailability of TACE in the underdeveloped Chinese mainland. In
view of these facts, we conducted a retrospective case-controlled
study to evaluate whether patients with unresectable HCC in the
Chinese mainland may benefit from TACE.
Materials and methods
Study population
A total of 129 patients with unresectable HCC who
were found to be eligible for this study were treated at the
Yijishang Hospital of Wannan Medical College between March, 2005
and June, 2007. The eligibility criteria for entering this study
were B or C class HCC according to the Barcelona Clinic Live Cancer
(BCLC) staging system (9), as
follows: (i) multinodular, performance status (PS) 0, Child-Pugh
class A–B; and (ii) portal invasion, N1, M1, PS 1–2, Child-Pugh
class A–B. Of the 129 patients, 102 received TACE as the case group
and 27 received symptomatic treatment alone as the control group.
The data retrieved from patient medical records included gender,
age of onset, levels of α-fetoprotein (AFP), BCLC staging and
Child-Pugh classification. This study was approved by the Research
and Ethics Committee of the Yijishan Hospital of Wannan Medical
College.
Treatment procedure
The control group received conservative treatment
alone for the management of the symptoms and complications. The
case group underwent transarterial Lipiodol chemoembolization
following a standard protocol. The femoral artery was catheterized
under local anaesthesia. Hepatic arteriography and superior
mesenteric arterial portovenography were performed to determine the
size and location of the tumor nodules. The right or left hepatic
artery feeding the tumor was super-selectively catheterized. An
emulsion of doxorubicin (30 mg/m2; Haizheng
Pharmaceutical Co., Ltd., Shanghai, China) or cisplatin (30
mg/m2; Haosen Pharmaceutical Company, Jiangxi, China)
mixed with Lipiodol (Guerbet, Villepinte, France) was infused prior
to mechanical obstruction. TACE was repeated every 1.5 to 3 months,
unless there was evidence of contraindications or progressive
disease.
Follow-up
Follow-up data were available for all the patients,
with a median follow-up of 11 months. The primary outcome measure
was survival, calculated from the date of diagnosis at the Yijishan
Hospital. The patients were followed up monthly at the outpatient
clinic or by telephone. During the follow-up period, 122 patients
succumbed to the disease and 7 patients were censored, 6 of which
were in the case group and 1 in the control group.
Statistical analysis
The comparison of the clinical characteristics
between the two groups was measured by the χ2 test. The
primary endpoint was survival, which was estimated with the
Kaplan-Meier method and the log-rank test. All the statistical
tests and P-values were two-tailed and P-values <0.05 were
considered to indicate statistically significant differences. All
the analyses were performed using the SPSS software, version 16.0
(SPSS Inc, Chicago, IL, USA).
Results
Patient characteristics
Between March, 2005 and June, 2007, a cohort of 129
patients with unresectable HCC entered this study. The case group
included 102 patients who received TACE and the control group
included 27 patients who received symptomatic treatment alone.
The clinical characteristics of the two groups are
summarized in Table I. None of
these characteristics, including age, gender, HBV infection status,
levels of AFP, Child-Pugh class and BCLC stage, were found to be
statistically significantly different between the TACE and the
symptomatic treatment groups.
 | Table IClinical characteristics of the TACE
and symptomatic treatment groups. |
Table I
Clinical characteristics of the TACE
and symptomatic treatment groups.
| Characteristics | No. | TACE n (%) | Symptomatic n
(%) | P-value n (%) |
|---|
| Total patient
no. | 129 | 102 | 27 | |
| Age (years) | | | | 0.230 |
| <60 | 61 | 51 (50.0) | 10 (37.0) | |
| ≥60 | 68 | 51 (50.0) | 17 (63.0) | |
| Gender | | | | 0.413 |
| Male | 108 | 84 (82.4) | 24 (88.9) | |
| Female | 21 | 18 (17.6) | 3 (11.1) | |
| Etiology | | | | 0.669 |
| HBV-positive | 113 | 90 (88.2) | 23 (85.2) | |
| HBV-negative | 16 | 12 (11.8) | 4 (14.8) | |
| AFP levels
(ng/ml) | | | | 0.459 |
| <400 | 78 | 60 (58.8) | 18 (66.7) | |
| ≥400 | 51 | 42 (41.2) | 9 (33.3) | |
| Child-Pugh class | | | | 0.280 |
| A | 92 | 75 (73.5) | 17 (63.0) | |
| B | 37 | 27 (26.5) | 10 (37.0) | |
| BCLC stage | | | | 0.317 |
| B | 100 | 81 (79.4) | 19 (70.4) | |
| C | 29 | 21 (20.6) | 8 (29.6) | |
Survival comparison
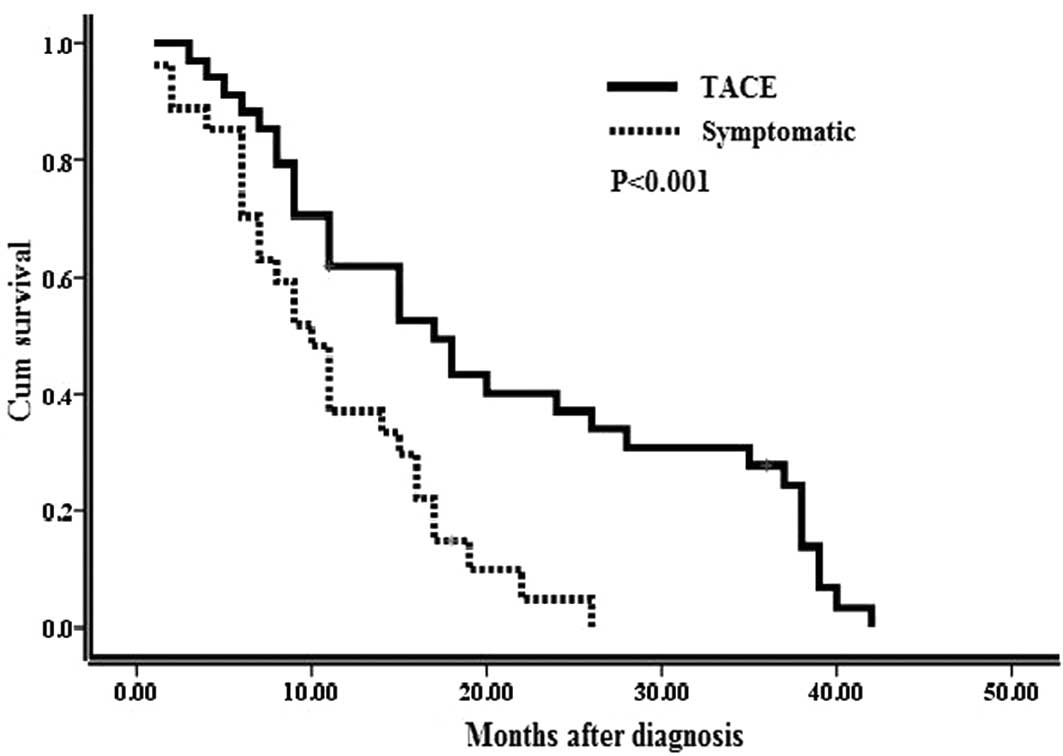
As shown in Fig. 1,
the TACE group exhibited a significantly better overall survival
compared to the symptomatic treatment group. The estimated 1-, 2-
and 3-year cumulative survival rates were 61.8, 34.0 and 24.3% for
the TACE group and 51.9, 9.9 and 0% for the symptomatic treatment
group (P<0.001).
Discussion
In the present study, we demonstrated that TACE was
significantly associated with improved survival compared to
symptomatic treatment in Chinese mainland patients with
unresectable HCC. There were no significant differences between the
two groups regarding clinical characteristics, such as Child-Pugh
class and BCLC stage.
There is currently no standard treatment for
patients with unresectable HCC, although the therapeutic approaches
are rapidly evolving, as the biology and natural history of this
disease becomes gradually elucidated (1). It is widely accepted that partial
hepatectomy, local ablation or liver transplantation offer the best
chance for long-term and disease-free survival for patients with
HCC. However, only a minority of patients with HCC are candidates
for these curative therapies, due to advanced tumor stage,
multicentric disease, poor liver function, or comorbidities at
diagnosis (10). Additionally,
systematic chemotherapy was shown to be mostly ineffective for HCC
(11).
In our study, we only evaluated the association of
TACE with survival, excluding assessment of the tumor response
following TACE, since tumor response in HCC patients was not found
to be a valuable predictor of prognosis (12).
A number of case-control and retrospective studies
on Western populations demonstrated a considerable improvement in
patient survival with TACE (13).
In a selective population with HCC, the 1-, 3- and 5-year survival
rates were 82, 47 and 26%, respectively (14). Despite these encouraging results,
there remains considerable controversy over the effectiveness and
safety of TACE. To date, there have been at least five randomized
controlled trials (RCTs) comparing TACE to symptomatic treatment by
survival (4–8). Three trials failed to demonstrate any
significant patient benefit from TACE regarding survival (4–6) and
two systematic reviews of non-randomized and randomized studies
also reported similar results, i.e., that TACE exerts little effect
on survival (15,16). By contrast, two subsequent RCTs
reported that TCAE significantly improved survival (7,8).
Accordingly, two more recent systematic reviews reached the same
positive conclusion (17,18). The results of our study are in
accordance with those of the two RCTs supporting that TCAE
significantly improved survival (7,8), one
of which was conducted in Hong Kong, China and included a patient
population similar to ours (8).
The inconsistence among these studies may be due to
several factors. First, the study populations were from different
sources. Three negative trials mainly included Western populations
or mixed-race patients with advanced tumors in a background of
alcohol-induced liver disease. There was a marked variation in the
outcome of patients with respect to race and ethnicity and the
natural history of untreated disease in European patients is
considerably better compared to that of Asians (19,20).
Our study, as well as the Hong Kong trial (8) mostly included patients positive for
HBV. It was reported that HBV-positive patients have a worse
prognosis (21). The 2-year
survival rate in the symptomatic group in our study was 9.9%, which
was lower compared to the 26% reported by a French multicenter
trial (6). In addition, the
selection criteria for patients receiving TACE must be considered.
Different tumor classifications were adopted in our study compared
with the three negative trials (4–6). Our
study adopted BLCL staging, whereas Okuda stage was used in the
three negative trials. Tumor stage was also significantly
associated with survival and may sway the effect of TACE (3).
Second, the technique and regimen of TACE may have
accounted, at least in part, for the inconsistence. Similar to the
two positive trials (7,8), in order to maximize the efficacy and
minimize toxicity, chemoembolization was conducted by selective
injection into the feeding artery in over half of our cases. In
comparison with the negative trials, our study used doxorubicin- or
cisplatin-Lipiodol emulsion. Among several single-agent
chemotherapies for HCC, doxorubicin was found to be the most
effective (22).
Furthermore, the negative systematic reviews and
meta-analyses may be outdated, as they excluded the two positive
trials. Subsequent systematic reviews, including the two positive
trials, demonstrated that TACE improved the survival of patients
with unresectable HCC (17,18).
In conclusion, our study confirmed that TACE is
efficient in prolonging survival in selected HCC patients compared
to symptomatic treatment alone. To the best of our knowledge, the
present study was the first case-control study to evaluate the
effect of TACE in Chinese mainland patients with HCC. The
limitations of our study lie in its retrospective nature. The
regimen and effect of TACE tailored for the Chinese population
require further investigation by large prospective RCTs conducted
in China.
References
|
1
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma. Hepatology. 42:1208–1236. 2005.
View Article : Google Scholar
|
|
3
|
Bruix J, Sala M and Llovet JM:
Chemoembolization for hepatocellular carcinoma. Gastroenterology.
127:S179–S188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pelletier G, Roche A, Ink O, Anciaux ML,
Derhy S, Rougier P, et al: A randomized trial of hepatic arterial
chemoembolization in patients with unresectable hepatocellular
carcinoma. J Hepatol. 11:181–184. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madden MV, Krige JE, Bailey S, Beningfield
SJ, Geddes C, Werner ID and Terblanche J: Randomised trial of
targeted chemotherapy with lipiodol and 5-epidoxorubicin compared
with symptomatic treatment for hepatoma. Gut. 34:1598–1600. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
No authors listed. A comparison of
lipiodol chemoembolization and conservative treatment for
unresectable hepatocellular carcinoma. Groupe d’Etude et de
Traitement du Carcinome Hépatocellulaire. N Engl J Med.
332:1256–1261. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Real MI, Montana X, et al;
Barcelona Liver Cancer Group. Arterial embolisation or
chemoembolisation versus symptomatic treatment in patients with
unresectable hepatocellular carcinoma: a randomised controlled
trial. Lancet. 359:1734–1739. 2002. View Article : Google Scholar
|
|
8
|
Lo CM, Ngan H, Tso WK, et al: Randomized
controlled trial of transarterial lipiodol chemoembolization for
unresectable hepatocellular carcinoma. Hepatology. 35:1164–1171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bruix J and Llovet JM: Prognostic
prediction and treatment strategy in hepatocellular carcinoma.
Hepatology. 35:519–524. 2002. View Article : Google Scholar
|
|
11
|
Zhu AX: Systemic treatment of
hepatocellular carcinoma: dawn of a new era? Ann Surg Oncol.
17:1247–1256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trevisani F, De Notariis S, Rossi C and
Bernardi M: Randomized control trials on chemoembolization for
hepatocellular carcinoma: is there room for new studies? J Clin
Gastroenterol. 32:383–389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lau WY, Yu SC, Lai EC and Leung TW:
Transarterial chemoembolization for hepatocellular carcinoma. J Am
Coll Surg. 202:155–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takayasu K, Arii S, Ikai I, et al; Liver
Cancer Study Group of Japan. Prospective cohort study of
transarterial chemoembolization for unresectable hepatocellular
carcinoma in 8510 patients. Gastroenterology. 131:461–469. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Simonetti RG, Liberati A, Angiolini C and
Pagliaro L: Treatment of hepatocellular carcinoma: a systematic
review of randomized controlled trials. Ann Oncol. 8:117–136. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mathurin P, Rixe O, Carbonell N, et al:
Review article: overview of medical treatments in unresectable
hepatocellular carcinoma - an impossible meta-analysis? Aliment
Pharmacol Ther. 12:111–126. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar
|
|
18
|
Camma C, Schepis F, Orlando A, et al:
Transarterial chemoembolization for unresectable hepatocellular
carcinoma: meta-analysis of randomized controlled trials.
Radiology. 224:47–54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chin PL, Chu DZ, Clarke KG, Odom-Maryon T,
Yen Y and Wagman LD: Ethnic differences in the behavior of
hepatocellular carcinoma. Cancer. 85:1931–1936. 1999. View Article : Google Scholar
|
|
20
|
Harrison LE, Reichman T, Koneru B, et al:
Racial discrepancies in the outcome of patients with hepatocellular
carcinoma. Arch Surg. 139:992–996. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Villa E, Moles A, Ferretti I, Buttafoco P,
Grottola A, Del Buono M, De Santis M and Manenti F: Natural history
of inoperable hepatocellular carcinoma: estrogen receptors’ status
in the tumor is the strongest prognostic factor for survival.
Hepatology. 32:233–238. 2000.PubMed/NCBI
|
|
22
|
Ganne-Carrie N and Trinchet JC: Systemic
treatment of hepatocellular carcinoma. Eur J Gastroenterol Hepatol.
16:275–281. 2004. View Article : Google Scholar
|