Introduction
Colorectal cancer is the third most common type of
cancer worldwide, the second leading cause of cancer-related
mortality in humans and the most common type of cancer in the
Western world. At the time of diagnosis, ∼30% of patients have
developed distant metastases, which predominantly occur in the
liver. Surgical removal of the tumor remains the only curative
approach (1,2). Of all affected patients ∼50% develop
liver metastases (3) and advanced
tumor stage with metastasis is among the main causes of the high
mortality rate. Over the last few years, the survival rates for
colorectal cancer have further increased due to multimodality
treatment concepts, particularly in Union for International Cancer
Control stage III and IV patients. In parallel to these modern
multimodality treatment concepts, novel and promising concepts,
including immunotherapeutic strategies, are actively being
investigated to further improve the clinical outcome.
The 5-year survival of patients undergoing hepatic
resection was reported to be ∼30%, compared with ∼10% among
patients without hepatic resection (4).
Ionizing radiation (IR) therapy is considered to be
an effective local cancer treatment, which eliminates cancer as
well as other cells within the tumor stroma. IR induces a variety
of DNA lesions, of which DNA double-strand breaks (DSBs) are the
most biologically important, since unrepaired or misre-paired DSBs
may lead to genomic instability and cell death. IR treatment
results in the activation of several DNA damage response molecules,
such as ataxia teleangiectasia mutated kinase (ATM), ataxia
teleangiectasia and Rad3-related protein (ATR) and catalytic
subunit of DNA-dependent protein kinase. ATM and ATR are large,
>300-kDa protein kinases that, upon activation, phosphorylate
numerous substrates and trigger repair or apoptosis, necrosis,
mitotic catastrophe and stress-induced premature senescence
(5–9).
Currently, applying nanocarriers for improving
cancer diagnostics and therapeutics poses emerging opportunities
and challenges (10,11). Liposomal drugs, such as pegylated
liposomes, may be designed to improve the pharmacological and
therapeutic index for cancer therapeutics. However, the limited
distribution of doxorubicin in solid tumors leads to drug
resistance, thus weakening the response to chemotherapy (12). There are considerable developments
on improving the therapeutic efficacy, reducing the side effects
and overcoming the drug resistance of multiplex nanoliposomes.
Internal radiotherapy with nanoliposomal (range, 100
nm) delivery of radionuclide or chemotherapeutic payloads may be
selectively targeted at the tumor, while reducing non-specific
accumulation (13). Rhenium-188
(188Re) emits a 155-keV γ-photon and a 2.12-MeV
β-particle suitable for nuclear imaging and targeted radionuclide
therapy. We previously investigated the biodistribution,
pharmacokinetics and single-photon emission computed
tomography/computed tomography imaging following intraperitoneal
and intravenous administration of 188Re-liposomes in C26
colon carcinoma ascites and solid tumor animal models (14,15).
Sorafenib is an orally available multikinase
inhibitor that targets Raf serine/threonine kinases (Raf-1,
wild-type B-Raf and B-Raf V600E), vascular endothelial growth
factor receptor (VEGFR)-1, -2 and -3, platelet-derived growth
factor receptor (PDGFR)-β and Flt3, c-Kit and p38 tyrosine kinases.
Sorafenib has a dual action that targets serine/threonine and
receptor tyrosine kinases, inhibiting i) the Raf cascade,
preventing the downstream mediation of cell growth and
proliferation; and ii) the VEGFR-2,-3/PDGFR-β signalling cascade,
inhibiting the activation of angiogenesis. Sorafenib acts by
inhibiting tumor growth and disrupting tumor microvasculature
through antiproliferative, antiangiogenic and proapoptotic effects
(16–19). Sorafenib has demonstrated
preclinical and clinical activity against several types of tumors,
such as renal cell, hepatocellular and colorectal carcinoma
(20–29).
Recent progress in the identification of master
tumori-genesis signaling pathways and protein kinases has led to
the development of novel targeted anticancer drugs. Sorafenib has
the potential to synergize with radiation through several
mechanisms, including proliferation inhibition of tumor cells,
vascular normalization of tumors and interference with
intracellular signaling pathways, which may affect the growth and
metastatic potential of tumors. Sorafenib administered in
combination with radiotherapy may eliminate more tumor cells. There
is a strong biological rationale to combining radiation with
sorafenib and it was effective in treating mice with metastatic
colorectal cancer (29,30). In this study, the tumor inhibitory
effect of 188Re-liposomes combined with sorafenib on
C26-luc metastatic colorectal liver tumours was
evaluated.
Materials and methods
Materials
The tungsten-188 (188W)/188Re
generator was purchased from Oak Ridge National Laboratory (Oak
Ridge, TN, USA). Elution of the 188W/188Re
generator with normal saline provided solutions of carrier-free
188Re as sodium perrhenate (NaReO4). The
pegylated liposome (Nano-X) was provided by Taiwan Liposome Company
(Taipei, Taiwan). N,N-bis
(2-mercaptoethyl)-N′,N′-diethylethylenediamine (BMEDA) was
purchased from ABX (Radeberg, Germany). Stannous chloride
(SnCl2) was purchased from Merck KGaA (Darmstadt,
Germany). Glucoheptonate (GH) powder was purchased from
Sigma-Aldrich (Bangalore, India). PD-10 column was purchased from
GE Healthcare (Uppsala, Sweden). All other chemicals were purchased
from Merck KGaA. RPMI-1640 cell culture medium and fetal bovine
serum (FBS) were purchased from Gibco (Carlsbad, CA, USA). Nexavar
was obtained from Bayer HealthCare Pharmaceuticals (Montville, NJ,
USA).
Cell cultures and animal model
The C26 murine colon carcinoma cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). This cell line was transfected with the luciferase gene as
reporter gene (C26-luc cells). The C26-luc cell line
stably expresses the firefly luciferase gene. C26-luc was
grown in RPMI-1640 medium supplemented with 10% (v/v) FBS and 2 mM
L-glutamine at 37°C in 5% CO2. Cells were detached with
0.05% trypsin/0.53 mM EDTA in Hanks′ balanced salt solution. Male
BALB/c mice were obtained from the National Animal Center of Taiwan
(Taipei, Taiwan), with food and water being provided ad
libitum in the animal house of the Institute of Nuclear Energy
Research (INER). The animal research protocols were approved by the
Institutional Animal Care and Use Committee (IACUC) at the
INER.
Liver metastasis model
A liver metastasis model was established in BALB/c
mice. The mice were anesthesized and a small incision was made
through the skin over the spleen after shaving. The spleen, visible
through the abdominal wall, was grasped and a small incision was
made over the tip. C26-luc cell suspension (30 μl) was
injected through a 29-gauge needle into the parenchyma of the
spleen. The spleen was removed 2 min later and the incision in the
skin was closed. Seven to ten days later, several metastases were
identified, often fused with one another.
Preparation of
188Re-liposomes
The labeling method of 188Re-liposomes
was as previously described (27–29).
Briefly, BMEDA and SnCl2 were used as the reductants and
GH was used as an intermediate ligand to form
188Re-SNS/S complexes. BMEDA (5 mg) were pipetted into a
glass vial. A volume of 0.5 ml of 0.17 mol/l GH dissolved in a 10%
acetate solution was added, followed by the addition of 120 μl (10
μg/μl) of SnCl2. After flushing the solution with
N2 gas, 188R of highly specific activity was
added. The vial was sealed and heated in water bath at 80°C for 1
h. The pegylated liposomes had an average particle size of
∼89.46±26.18 nm. Nano-X pegylated liposomes (1 ml) were added to
the 188Re-BMEDA (600–740 MBq) solution and incubated at
60°C for 30 min. 188Re-liposomes were separated from
free 188Re-BMEDA using an PD-10 column (GE Healthcare)
eluted with normal saline. Each 0.5-ml fraction was collected into
a tube. The opacity of pegylated liposomes was employed to visually
monitor the collection of 188Re-liposomes. The labeling
efficiency was determined using the activity in pegylated liposomes
after separation divided by the total activity prior to
separation.
Therapeutic efficacy
Treatment was initiated 7-10 days after intrasplenic
cell inoculation. A total of 32 BALB/c C26-luc tumor-bearing
mice were randomly divided into four groups, (n=8 per group) and
one group was randomly selected as the control. To confirm the
metastasis of tumor cells to the liver, liver tissue was isolated
on day 10 post-implantation and ex vivo images were
captured. Single-dose treatments with 188Re-liposomes
were performed on day 1 and triple-dose treatments with Nexavar (10
mg/kg) were performed once every other day for one week on days 3,
5 and 7. Bioluminescence images were captured on days 1 and 15.
Prior to the in vivo imaging, the mice were anesthetized
with isoflurane. D-luciferin solution was subsequently injected
intraperitone-ally (150 mg/kg). The mice were imaged using a
Xenogen IVIS® 100 small animal imaging system (Caliper
Life Sciences, Hopkinton, MA, USA). Excitation
(λex=710–760 nm) and emission (λem=810–875
nm) filters were used. Identical illumination settings, including
exposure time (10 sec), binning factor (8), f-stop (1) and fields of view (25×25 cm), were
used for all image acquisitions. Fluorescent and photographic
images were acquired and merged. The images were acquired and
analyzed using Living Image® 2.0 software (Caliper Life
Sciences). The fluorescence signal intensity of the abdominal
region was quantified by creating an circular region of interest
(ROI) using Living Image® 2.0 software.
Results
Labeling efficiency of
188Re-liposomes
The encapsulation efficiency of
188Re-BMEDA in pegylated nanoliposomes was 79.2±3.7%.
The radiochemical purity of 188Re-liposomes exceeded
95%. The average particle size of 188Re-liposomes was
similar to that prior to 188Re-BMEDA encapsulation.
Bioluminescence imaging for monitoring
therapeutic response
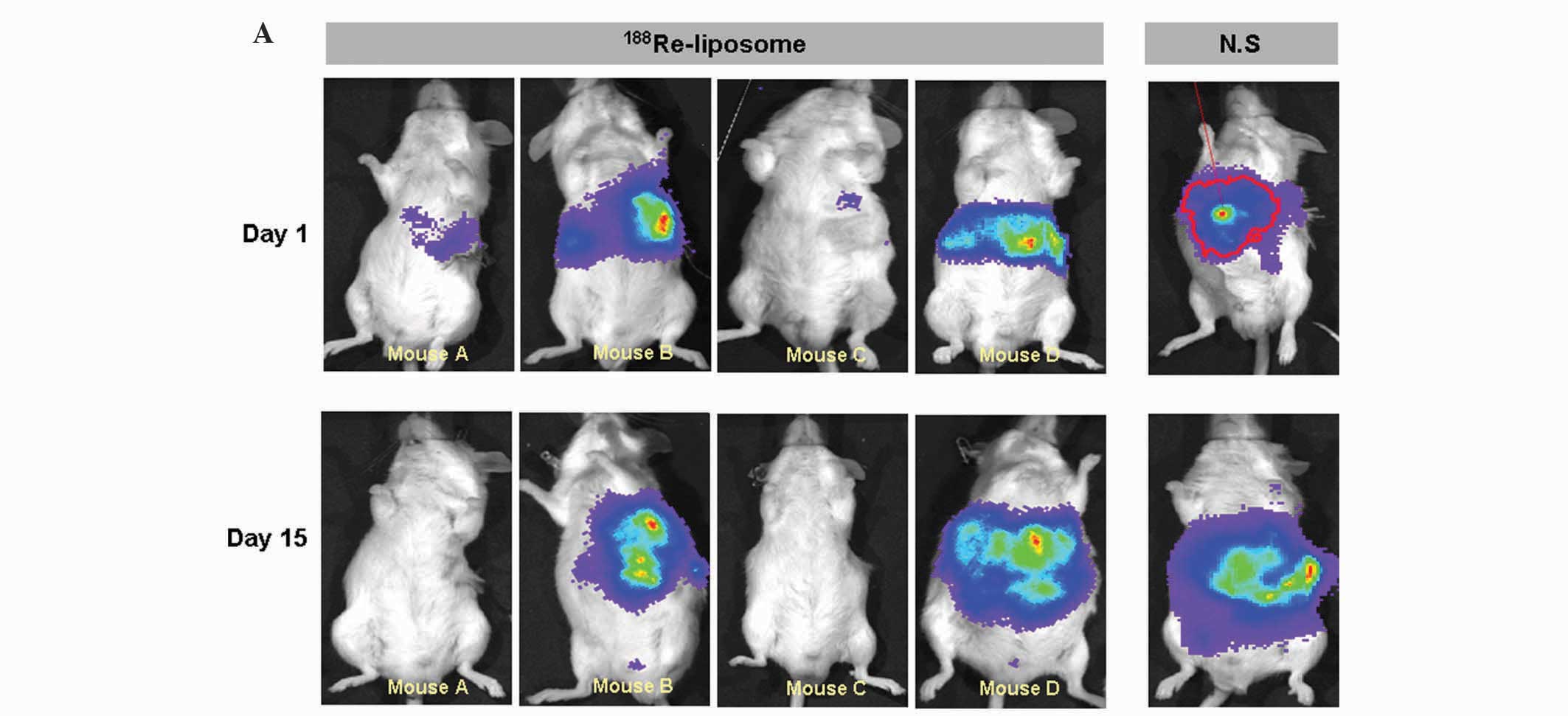
The therapeutic responses were monitored by
bioluminescence imaging prior to and twice a week following drug
treatment (Fig. 1A). Significant
suppression of tumor growth was observed with the use of
188Re-liposomes. The most significant tumor inhibition
was achieved with the combination therapy using sorafenib followed
by radio-therapy with 188Re-liposomes. In this study,
the normal saline group was used as control for comparison
purposes. The photon counts from the bioluminescence imaging were
collected and measured from the ROIs of the tumor sites. The mean
photon flux of all the treatments correlated with tumor size. The
results demonstrated that the mean photon flux of the control group
increased rapidly (2.2×108±1.4×108 ph/sec)
compared with the group treated with 188Re-liposomes
(4.0×107±2.1×107 ph/sec) at day 15 after
treatment. The mean photon fluxes, as a function of time after
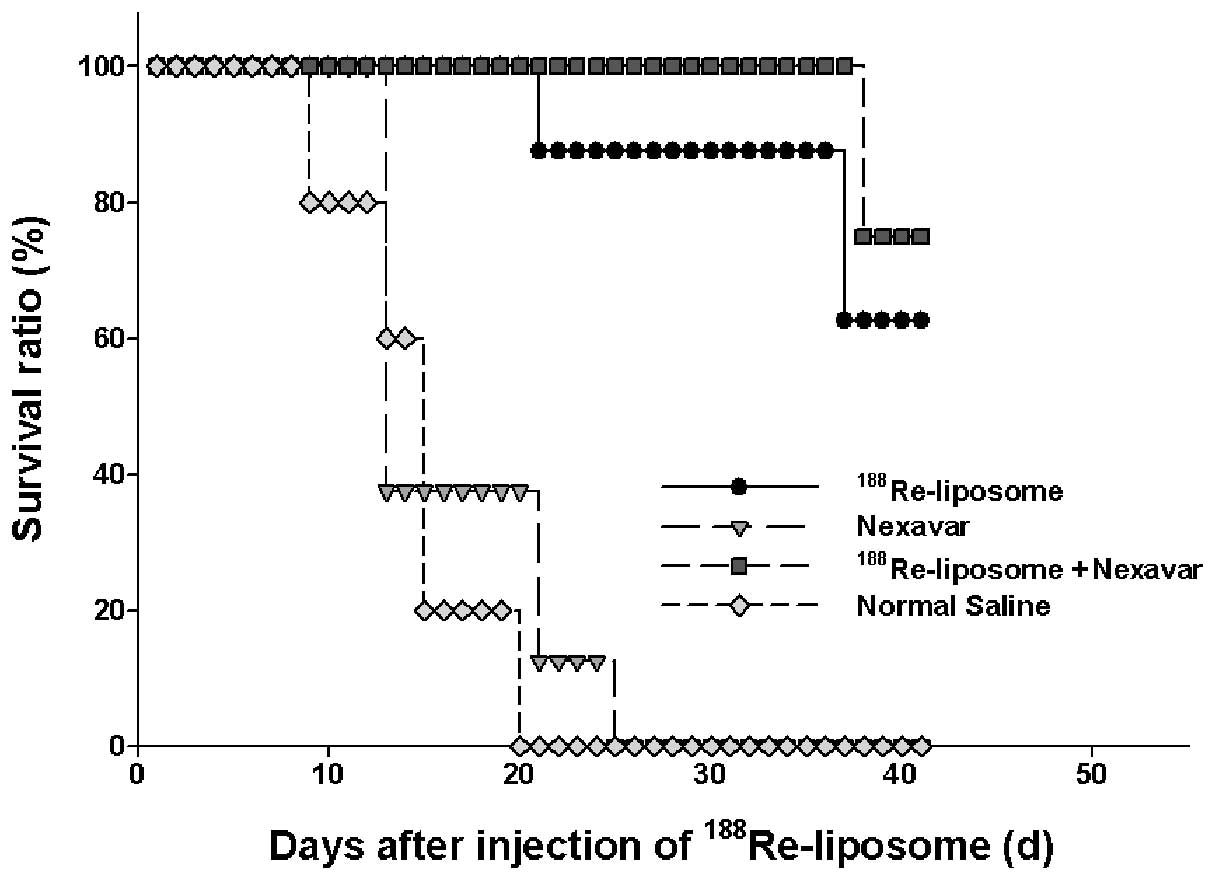
initiation of the various treatments, are shown in Fig. 1B and the survival curves for the
different treatment groups are compared in Fig. 2. At the end of the experiment (41
days after therapeutics administration), 6 mice (75%) treated with
188Re-liposomes plus sorafenib (P=0.000) and 5 mice
(62.5%) treated with 188Re-liposomes alone (P=0.000)
remained alive. These results confirm that, among all treatments,
the greatest tumor control was achieved by the combination of
radiotherapy and chemotherapy.
Discussion
Sorafenib is hypothesized to affect tumor growth by
directly inhibiting tumor cell proliferation, promoting apoptosis
and inhibiting tumor angiogenesis, leading to tumor stasis with
occasional tumor regressions. This mechanism of action usually
precludes drugs such as sorafenib as single-agent treatment for the
majority of solid tumors, since optimal benefits are achieved when
combined with conventional chemotherapeutic agents and/or
radiotherapy. The combination of sorafenib with radiation was
previously described in a variety of human tumor cell lines in
vitro and in vivo. Plastaras et al (30) observed that sorafenib exhibits a
broad range of antigrowth activity in viability assays in several
human tumor cell lines and may also selectively induce apoptosis in
some of these cell lines. Sorafenib slows cell cycle progression
and prevents irradiated cells from reaching and accumulating at
G2-M phase. Radiation treatment followed sequentially by sorafenib
was found to be associated with the greatest tumor growth delay
(30), whereas concurrent
treatment with radiation and sorafenib was not superior to
radiation alone. In our study, the group of
188Re-liposome treatment followed sequentially by
sorafenib was found to achieve a higher survival rate compared with
the 188Re-liposome only, sorafenib only and normal
saline control groups.
IR is used as a primary treatment for several types
of cancer. Exposure of carcinoma cells to low doses of IR was shown
to cause DNA damage and rapid activation of p53, ATM, ATM- and
Rad3-related proteins, which further activate growth factor
receptors in the plasma membrane (31–34).
The ATM/p53 pathway, the mitogen-activated protein kinase (MAPK)
cascade and the nuclear factor κ-light-chain-enhancer of activated
B cells (NF-κB) pathway are some of the pathways that are activated
in response to radiation, affecting long-term cell survival. Cell
signaling through the MAPK pathway may result in the expression of
cyclin D1 and cell cycle progression through the G1/S checkpoint.
Cyclin D1 is a component of the core cell cycle machinery.
Abnormally high levels of cyclin D1 are detected in several types
of human cancer (35,36). Kim et al (23) reported that exposure of colon
cancer cells to sorafenib combined with irradiation resulted in
increased radiation-induced cytotoxicity. While radiation induced
the expression of cyclin B1, sorafenib inhibited cyclin B1
expression. Sorafenib also attenuated cyclin B1 expression when
combined with radiation. Sorafenib was shown to inhibit cell cycle
progression via the downregulation of cyclin B1, leading to failure
of the cells to undergo the transition from the G2 to the M phase.
The combination of radiation with sorafenib was shown to reinforce
radiation-induced mitotic arrest by attenuating cyclin B1 (23).
In a study conducted by Plastaras et al
(30), HCT116 tumor-bearing mice
were irradiated with four fractions of 3 Gy/day, followed by 7 days
of 60 mg/kg/day sorafenib and it was observed that radiation
treatment followed sequentially by sorafenib achieved a more
significant tumor growth delay compared to radiation alone or
concurrent treatment (30). Suen
et al (22) investigated
the combination effect of sorafenib and radiation using two human
colorectal cancer cell lines, HT29 and SW48, and observed that
radiation treatment followed sequentially by sorafenib treatment
exhibited synergistic cytotoxicity in HT29/tk-luc cells,
with increased tumor cell apoptosis. NF-κB activation induced by
radiation may be reduced by sorafenib (22). Kuo et al (27) reported that the combination of
sorafenib and radiation achived the maximum tumor growth inhibition
compared to sorafenib alone or radiation alone. Sorafenib and
radiation act synergistically in the treatment of human colorectal
carcinoma. This synergistic action is mediated through the
inhibition of radiation-induced NF-κB expression and its regulated
downstream gene products (27). In
this study, the C26-luc tumor-bearing mice were treated once
every other day for 1 week with 10 mg/kg sorafenib by gavage 24 h
after 188Re-liposome treatment and were continuously
treated for 1 week post-irradiation. The results demonstrated that
the optimal tumor growth control and survival ratio was achieved
with the combination treatment vs. sorafenib alone or radiation
alone. Radiation activates the DNA binding of NF-κB and results in
the increase of cyclin D1 and cyclin B1, an effect which is
suppressed by sorafenib. Therefore, the sequential administration
of sorafenib may be an effective cancer treatment schedule when
combined with radiation treatment.
Acknowledgements
The authors would like to thank all
members of the research committee for their valuable support during
this research.
References
|
1.
|
Reissfelder C, Timke C, Schmitz-Winnenthal
H, et al: A randomized controlled trial to investigate the
influence of low dose radiotherapy on immune stimulatory effects in
liver metastases of colorectal cancer. BMC Cancer. 11:4192011.
View Article : Google Scholar
|
|
2.
|
Reissfelder C, Rahbari NN, Koch M, Ulrich
A, Pfeilschifter I, Waltert A, Muller SA, Schemmer P, Buchler MW
and Weitz J: Validation of prognostic scoring systems for patients
undergoing resection of colorectal cancer liver metastases. Ann
Surg Oncol. 16:3279–3288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
4.
|
Cummings LC, Payes JD and Cooper GS:
Survival after hepatic resection in metastatic colorectal cancer: a
population-based study. Cancer. 109:718–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Vavrova J and Rezacova M: The importance
of senescence in ionizing radiation-induced tumour suppression.
Folia Biol. 57:41–46. 2011.PubMed/NCBI
|
|
6.
|
Bakkenist CJ and Kastan MB: DNA damage
activates ATM through intermolecular autophosphorylation and dimer
dissociation. Nature. 421:499–506. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Jeggo PA, Geuting V and Lobrich M: The
role of homologous recombination in radiation-induced double-strand
break repair. Radiother Oncol. 101:7–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lobrich M and Jeggo PA: The impact of a
negligent G2/M checkpoint on genomic instability and cancer
induction. Nat Rev Cancer. 7:861–869. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jeggo PA and Löbrich M: DNA double-strand
breaks: their cellular and clinical impact? Oncogene. 26:7717–7719.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Davis ME, Chen ZG and Shin DM:
Nanoparticle therapeutics: an emerging treatment modality for
cancer. Nat Rev Drug Discov. 7:771–782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Cho K, Wang X, Nie S, Chen ZG and Shin DM:
Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer
Res. 14:1310–1316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wolpin BM, Meyerhardt JA, Mamon HJ and
Mayer RJ: Adjuvant treatment of colorectal cancer. CA Cancer J
Clin. 57:168–185. 2007. View Article : Google Scholar
|
|
13.
|
Brannon-Peppas L and Blanchette JO:
Nanoparticle and targeted systems for cancer therapy. Adv Drug
Deliv Rev. 56:1649–1659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chang YJ, Chang CH, Chang TJ, Yu CY, Chen
LC, Jan ML, Luo TY, Lee TW and Ting G: Biodistribution,
pharmacokinetics and microSPECT/CT imaging of
188Re-bMEDA-liposome in a C26 murine colon carcinoma
solid tumor animal model. Anticancer Res. 27:2217–2225.
2007.PubMed/NCBI
|
|
15.
|
Chen LC, Chang CH, Yu CY, Chang YJ, Hsu
WC, Ho CL, Yeh CH, Luo TY, Lee TW and Ting G: Biodistribution,
pharmacokinetics and imaging of 188Re-BMEDA-labeled
pegylated liposomes after intraperitoneal injection in a C26 colon
carcinoma ascites mouse model. Nucl Med Biol. 34:415–423. 2007.
|
|
16.
|
Ibrahim N, Yu Y, Walsh WR and Yang JL:
Molecular targeted therapies for cancer: Sorafenib mono-therapy and
its combination with other therapies (Review). Oncol Rep.
27:1303–1311. 2012.PubMed/NCBI
|
|
17.
|
Dal Lago L, D′Hondt V and Awada A:
Selected combination therapy with sorafenib: a review of clinical
data and perspectives in advanced solid tumors. Oncologist.
13:845–858. 2008.PubMed/NCBI
|
|
18.
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Escudier B, Eisen T, Stadler WM, et al
TARGET Study Group: Sorafenib in advanced clear-cell renal-cell
carcinoma. New Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Llovet JM, Ricci S, Mazzaferro V, et al
SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. New Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Suen AW, Galoforo S, Marples B, McGonagle
M, Downing L, Martinez AA, Robertson JM and Wilson GD: Sorafenib
and radiation: a promising combination in colorectal cancer. Int J
Radiat Oncol Biol Phys. 78:213–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kim YB, Jeung HC, Jeong I, Lee K, Rha SY,
Chung HC and Kim GE: Mechanism of enhancement of radiation-induced
cytotoxicity by sorafenib in colorectal cancer. J Radiat Res.
54:52–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Di Nicolantonio F, Martini M, Molinari F,
Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L,
Frattini M, Siena S and Bardelli A: Wild-type BRAF is required for
response to panitumumab or cetuximab in metastatic colorectal
cancer. J Clin Oncol. 26:5705–5712. 2008.
|
|
25.
|
Ratain MJ, Eisen T, Stadler WM, et al:
Phase II placebo-controlled randomized discontinuation trial of
sorafenib in patients with metastatic renal cell carcinoma. J Clin
Oncol. 24:2505–2512. 2006. View Article : Google Scholar
|
|
26.
|
Wehler TC, Hamdi S, Maderer A, et al:
Single-agent therapy with sorafenib or 5-FU is equally effective in
human colorectal cancer xenograft - no benefit of combination
therapy. Int J Colorect Dis. 28:385–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kuo YC, Lin WC, Chiang IT, Chang YF, Chen
CW, Su SH, Chen CL and Hwang JJ: Sorafenib sensitizes human
colorectal carcinoma to radiation via suppression of NF-kappaB
expression in vitro and in vivo. Biomed Pharmacother. 66:12–20.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Martinelli E, Troiani T, Morgillo F, et
al: Synergistic antitumor activity of sorafenib in combination with
epidermal growth factor receptor inhibitors in colorectal and lung
cancer cells. Clin Cancer Res. 16:4990–5001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Galal KM, Khaled Z and Mourad AM: Role of
cetuximab and sorafenib in treatment of metastatic colorectal
cancer. Indian J Cancer. 48:47–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Plastaras JP, Kim SH, Liu YY, et al: Cell
cycle dependent and schedule-dependent antitumor effects of
sorafenib combined with radiation. Cancer Res. 67:9443–9454. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Amundson SA, Bittner M and Fornace AJ Jr:
Functional genomics as a window on radiation stress signaling.
Oncogene. 22:5828–5833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Abraham RT: Checkpoint signaling:
epigenetic events sound the DNA strand-breaks alarm to the ATM
protein kinase. Bioessays. 25:627–630. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Valerie K, Yacoub A, Hagan MP, Curiel DT,
Fisher PB, Grant S and Dent P: Radiation-induced cell signaling:
inside-out and outside-in. Mol Cancer Ther. 6:789–801. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Dent P, Yacoub A, Fisher PB, Hagan MP and
Grant S: MAPK pathways in radiation responses. Oncogene.
22:5885–5896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Deshpande A, Sicinski P and Hinds PW:
Cyclins and cdks in development and cancer: a perspective.
Oncogene. 24:2909–2915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Jirawatnotai S, Hu Y, Michowski W, et al:
A function for cyclin D1 in DNA repair uncovered by protein
interactome analyses in human cancers. Nature. 474:230–234. 2011.
View Article : Google Scholar : PubMed/NCBI
|