Introduction
Chemotherapy-induced nausea and vomiting (CINV) is
an adverse event that significantly impairs the patients’ quality
of life (1). Thus, to ensure the
continuity of chemotherapy, it is crucial to provide appropriate
supportive care to prevent CINV.
With regard to preventing CINV, antiemetic agents
corresponding to each emetogenic risk have been recommended in
antiemetic guidelines. Novel antiemetics, such as aprepitant, a
selective neurokinin-1 receptor antagonist (NK1RA) and
palonosetron, a long-acting second-generation serotonin
(5-HT3) receptor antagonist (5-HT3RA), were
relatively recently developed. Consequently, the guidelines of the
American Society of Clinical Oncology (2), the National Comprehensive Cancer
Network (3) and the Multinational
Association for Supportive Care in Cancer (4) were updated to incorporate aprepitant
and palonosetron and their use as antiemetics was recommended,
corresponding to either high or moderate emetic risk.
Additionally, in Japan, the antiemetic guidelines
issued by the Japan Society of Clinical Oncology (JSCO guidelines)
(5) recommend two-drug
combinations of a 5-HT3RA and dexamethasone for use in
moderately emetogenic chemotherapy (MEC) and three-drug
combinations of a 5-HT3RA, dexamethasone and
NK1RA for use in highly emetogenic chemotherapy
(HEC).
The symptoms of nausea and vomiting are categorized
as either acute-phase, defined as episodes occurring within 24 h of
the administration of chemotherapy, or delayed-phase, defined as
episodes occurring after 24 h (6,7). The
development of granisetron, a first-generation 5-HT3RA,
was shown to mitigate acute nausea and vomiting (8), although its efficacy for delayed
nausea and vomiting is limited (9). However, the more recently developed
aprepitant (10) and palonosetron
(11) have demonstrated promising
outcomes in the control of acute- and delayed-phase nausea and
vomiting.
In the JSCO guidelines, there is a paragraph
highlighting the need to consider the evidence-based proper use of
anti-emetics upon correctly evaluating the emetogenic risks of each
agent. However, the frequency of CINV is greatly dependent on the
type and combination of chemotherapeutic agents, which requires
further investigation.
Thus, in an attempt to assess the efficacy of the
currently available antiemetic agents for nausea and vomiting
following standard chemotherapy, we conducted a prospective study
through the use of patient diaries on nausea and vomiting.
Materials and methods
Population
In the present study, participants were recruited
among patients receiving HEC or MEC in the Ambulatory Therapy
Center of our institution between August, 2010 and March, 2011.
Patients with episodes of vomiting within 24 h prior to the
administration of chemotherapy, those who required the
administration of HEC or MEC on or after day 2 and those who
received radiation therapy were excluded from the study.
The study protocol was approved by the Institutional
Review Board prior to the initiation of the study and all the
patients provided written informed consent prior to enrollment.
Assessment of antiemetic efficacy and
safety
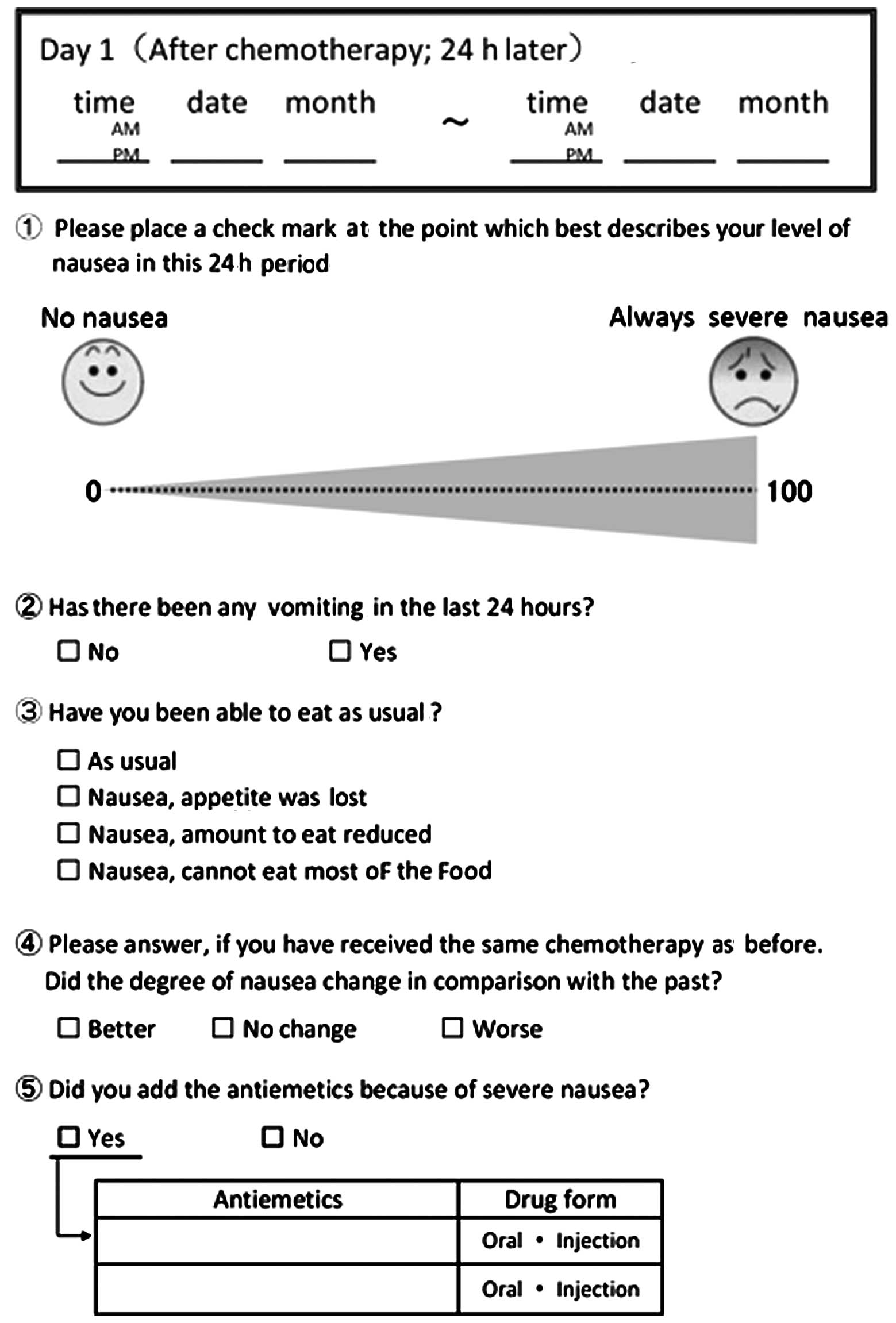
The episodes of nausea and vomiting were assessed
through the use of patient diaries from the day of the treatment
until day 5 (Fig. 1). The efficacy
endpoints were defined as follows: Complete response (CR), no
emetic episodes and no rescue therapy; and total control (TC), no
emetic episodes, no rescue therapy, no nausea and no appetite loss.
The patients’ diaries, in which the scores on the visual analogue
scale (VAS) for assessing the severity of nausea (12,13),
the presence of vomiting episodes and the appetite levels were
recorded by the patients themselves over a period of 5 days, were
collected and assessed. The appetite levels were recorded with a
four-grade assessment system: Normal, appetite diminished due to
nausea, food portions decreased due to nausea and almost no food
intake due to nausea.
Statistical analysis
In order to compare the assessments of antinausea or
antiemetic activity, a statistical analysis was performed using the
χ2 test. The level of significance was set at 0.05 for
all the tests. All the statistical analyses were performed using
JMP software, version 9.0.2 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
The characteristics of the 103 patients are shown in
Table I. The patients included 41
men and 62 women, with a median age of 61.6 years (range, 36–81
years). A total of 42 patients received HEC and 61 patients
received MEC. The tumor types included colorectal (45), breast
(24), gynecological (9), lung (8), biliary tract (6), gastric (6)
and other types of cancer (5). The chemotherapeutic regimens used
were as follows: for HEC, fluorouracil + epirubicin +
cyclophosphamide; cisplatin (CDDP) + gemcitabine; or CDDP +
irinotecan (CPT-11); and for MEC, capecitabine +
oxaliplatin/folinic acid + fluorouracil + oxiplatin ± bevacizumab
(Bev); folinic acid + fluorouracil + irinotecan
(FOLFIRI)/irinotecan + S-1 (IRIS) ± Bev; or CBDCA + paclitaxel. The
four agents used as a 5-HT3RA were granisetron
hydrochloride (granisetron), azasetron hydrochloride (azasetron),
ramosetron hydrochloride (ramosetron) and palonosetron
hydrochloride (palonosetron). One of these 5-HT3RAs plus
dexamethasone and aprepitant (a three-drug combination) was
administered to 42 patients.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | Patient no.
(n=103) |
|---|
| Median age, years
(range) | 61.6 (36–81) |
| Tumor type | |
| Colorectal | 45 |
| Breast | 24 |
| Gynecological | 9 |
| Lung | 8 |
| Biliary | 6 |
| Gastric | 6 |
| Other | 5 |
| Gender | |
| Male | 41 |
| Female | 62 |
| Chemotherapy
regimen | |
| HEC (n=42)
FEC/CDDP + CPT-11/CDDP + GEM/other | 21/9/3/9 |
| MEC (n=61) CBDCA +
PTX/Xelox ± Bev/FOLFOX ± Bev/FOLFIRI/IRIS + Bev/other | 12/22/15/4/1/7 |
| Prescription of
antiemetics | |
|
Dexamethasone+5-HT3RA+NK1RA | 45 |
|
Dexamethasone+5-HT3RA | 54 |
| 5-HT3RA
alone | 4 |
| 5-HT3RAs
(HEC/MEC) | |
| Granisetron
(n=55) | 10/45 |
| Ramosetron
(n=15) | 11/4 |
| Palonosetron
(n=29) | 16/13 |
| Azasetron
(n=4) | 4/0 |
| NK1RA and
non-NK1RA (HEC/MEC) | |
| NK1RA
(n=45) | 32/13 |
|
Non-NK1RA (n=58) | 10/48 |
Efficacy
The CR and TC rates in the delayed phase were
assessed for all the patients and for those receiving HEC and MEC,
by comparing the patients administered palonosetron (group P) to
those administered a different 5-HT3RA (group X)
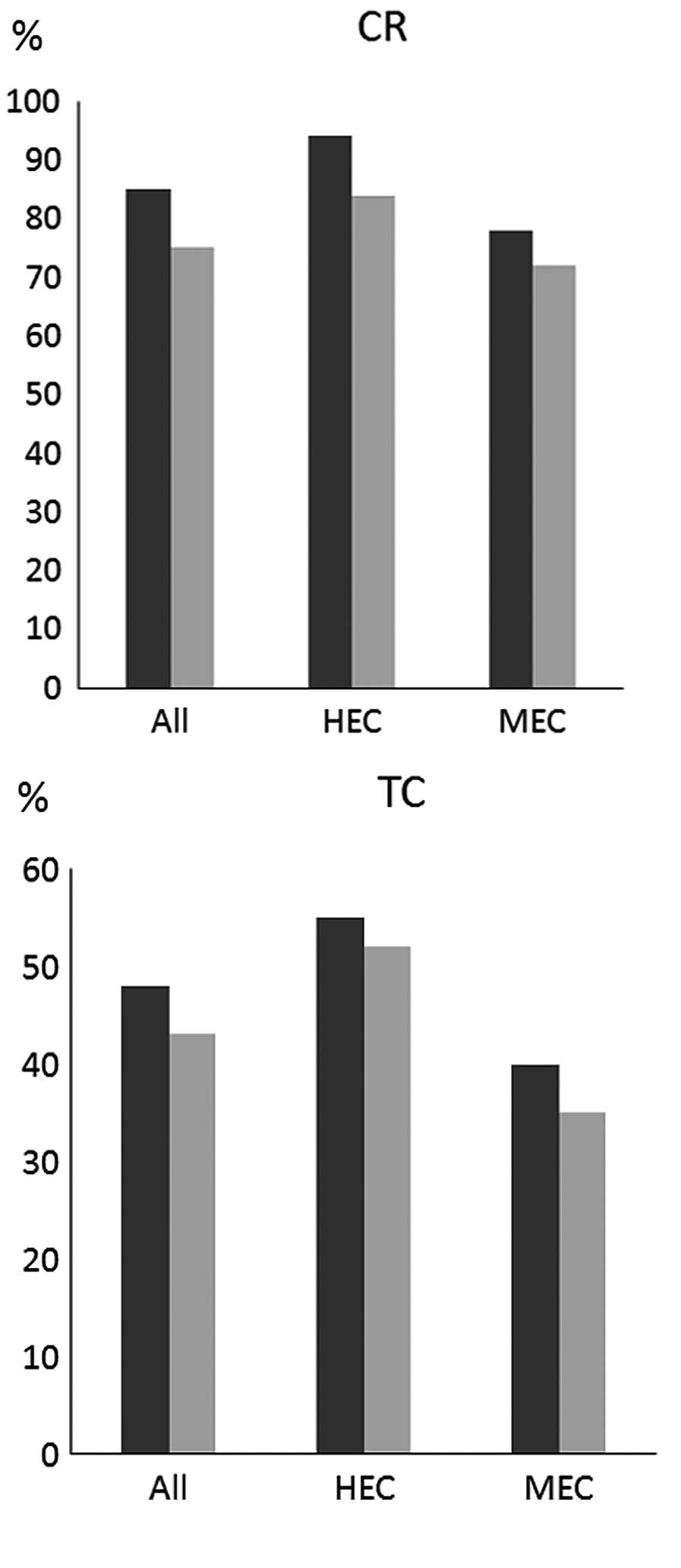
(Fig. 2). The CR rates for all,
HEC and MEC patients in group P vs. those in group X were 86 vs.
76%, 93 vs. 84% and 77 vs. 72%, respectively. The TC rates for all,
HEC and MEC patients in group P vs. those in group X were 48 vs.
43%, 55 vs. 52% and 40 vs. 35%, respectively. Although both groups
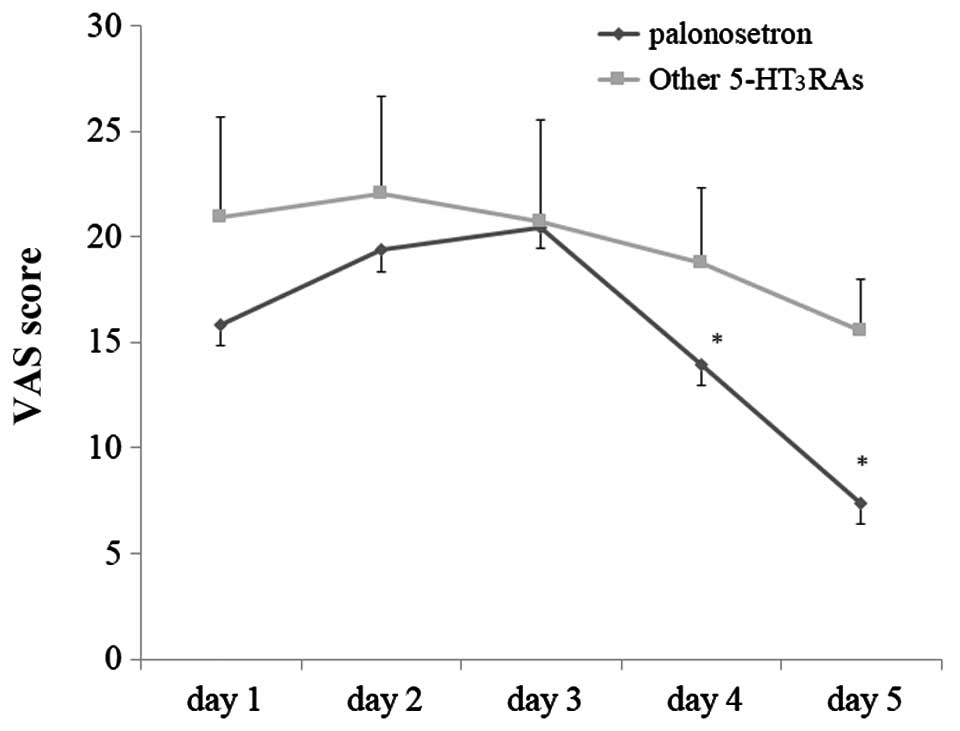
exhibited an improvement in the VAS scores in the delayed phase
over time (Fig. 3), the changes
exhibited by group P patients were more prominent compared to those
exhibited by group X patients.
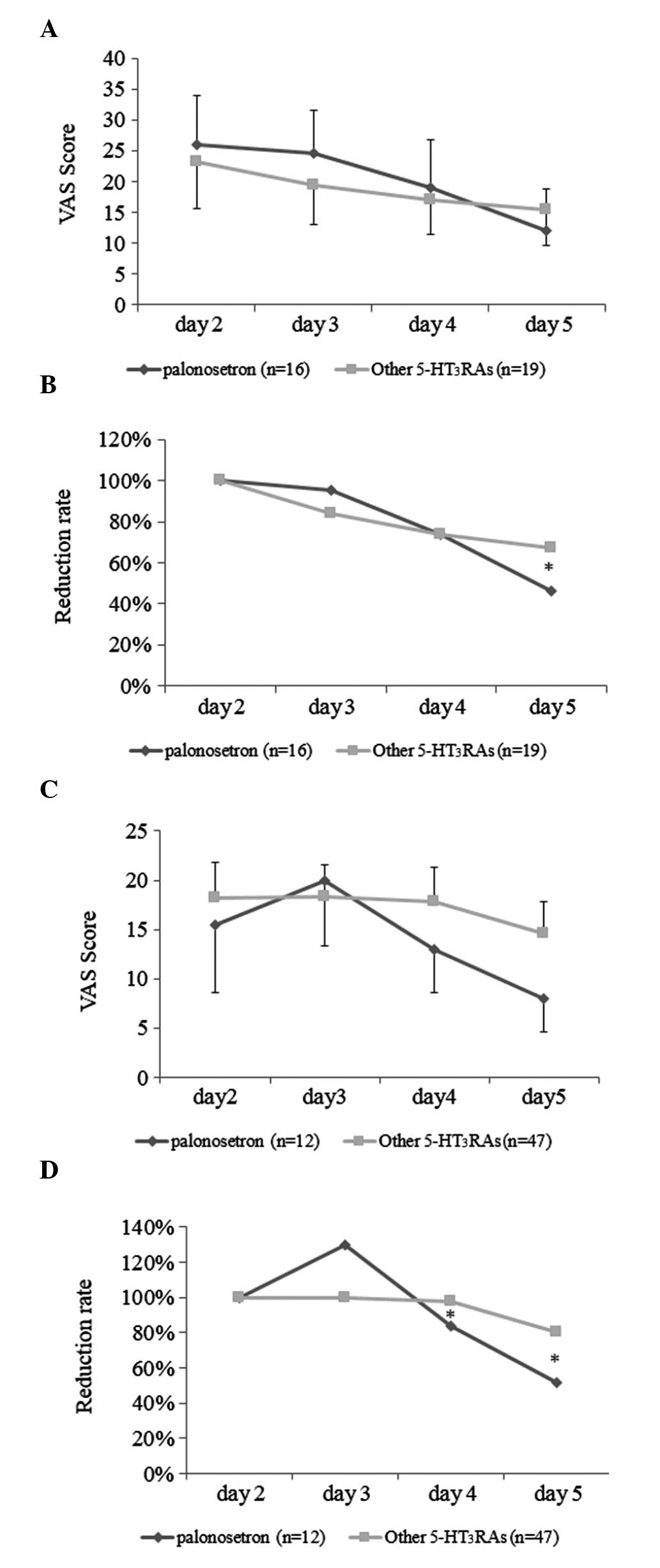
The changes in the VAS scores in the delayed phase
(days 2–5) in HEC and MEC patients were further assessed. In HEC
patients, the VAS scores on day 5 were lower in group P patients
compared to those in group X patients (Fig. 4A). When the reduction in the VAS
scores on days 3–5 in HEC was assessed by defining the scores of
day 2 as 100%, the decrease in VAS scores on day 5 in group P
patients was significantly more prominent, with a more significant
improvement compared to that in group X patients (Fig. 4B). Furthermore, as regards MEC
patients, the VAS scores in group P were also lower compared to
those in group X on days 4 and 5 (Fig.
4C); when the relative reduction in VAS scores in group P
patients after day 2 was assessed, the decrease in VAS scores on
days 4 and 5 in group P patients was significantly more prominent
compared to that in group X patients (Fig. 4D).
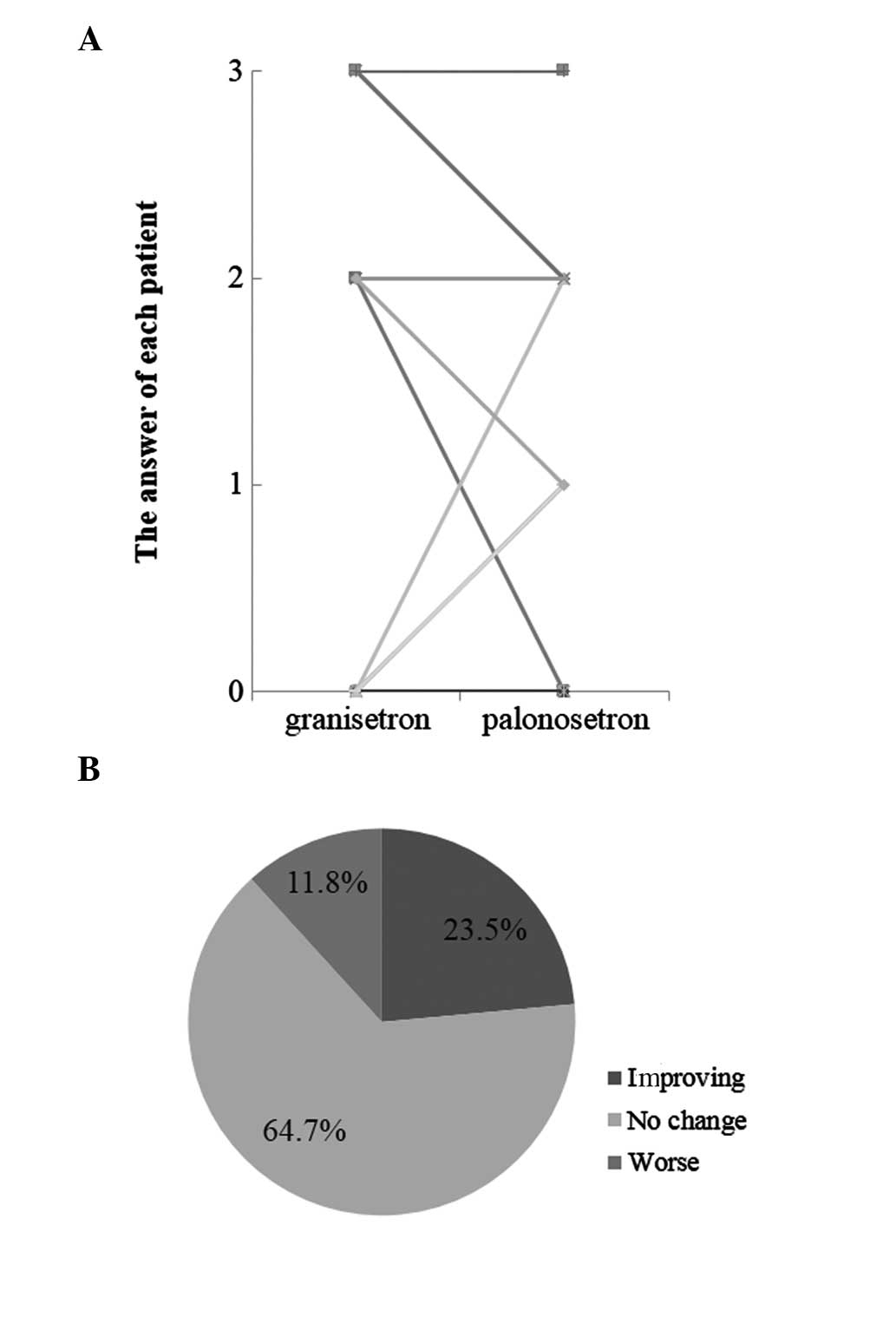
Furthermore, changes in food intake were assessed in
18 patients in whom granisetron was switched to palonesetron
(Fig. 5). In the delayed phase, a
total of 22.2% (4/18) of the patients attained increased food
intake and exhibited improved appetite.
Discussion
5-HT3RAs, NK1RAs and
dexamethasone are effective anti-emetic agents used to prevent
CINV. In Japan, NK1RAs are currently covered by public
health insurance and palonosetron has become available as a
second-generation 5-HT3RA. These antiemetics are also
recommended in guidelines published in the USA and Europe (2–4).
The efficacy of palonosetron was demonstrated in the
present study. Palonosetron exhibited a greater efficacy compared
to other conventional 5-HT3RAs, particularly on days 4
and 5, as it has a long half-life (14); it was also found to be effective in
HEC and MEC in the delayed phase. In certain cases in the present
study, granisetron was replaced by palonosetron in an attempt to
achieve higher efficacy of the newly developed antiemetics to
determine the effects of chemotherapy on food intake. Improvements
in appetite were previously reported (11), particularly in the delayed phase,
in one-quarter of the patients in whom the antiemetic agent was
switched to palonosetron. There were no serious side effects and,
although there has not yet been a listing of palonosteron in the
Japanese guidelines (5), it was
suggested that the use of palonosetron as a 5-HT3RA be
prioritised over that of other available 5-HT3RAs.
Additionally, improvement of the VAS scores on days
4 and 5 is crucial in terms of maintaining the nutritional status
and reducing the time of appetite loss. However, there were certain
limitations to our study. When CR and TC rates in the delayed phase
were assessed by HEC and MEC regimens, the TC rates for both groups
were low; in particular, the TC rates in MEC were 33%, which is
lower compared to the 51.9% previously reported (15). This discrepancy may be attributed
to the TC rate in the present study being more strictly defined
[i.e., complete control (CC) was previously defined as ‘no nausea
and no vomiting’]. The other plausible cause for these
discrepancies may be the fact that there were some cases in which
dexamethasone was not administered on days 2 or 3. In fact, in
those cases receiving the guideline-directed regimens, the CC rates
were almost equivalent to those previously reported (data not
shown). The detailed mechanism through which dexamethasone
mitigates nausea and vomiting has not been fully elucidated
(16); however, it is an
evidence-based agent reported to exhibit high dose-dependent
efficacy (17). The present study
demonstrated that the CR and TC rates were decreased in the
corresponding dexamethasone-free regimens, indicating that
guideline-directed antiemetic therapies should be recommended.
However, the development of dexamethasone-free regimens is required
for patients who are unable to tolerate the adverse effects of
dexamethasone, including hyperglycemia and insomnia (18,19).
The JSCO guidelines recommend the use of two-drug
combinations for antiemetic regimens against CINV in MEC (20); however, by definition, the
frequency of CINV episodes in MEC is 30–90% (21) and the addition of aprepitant to
certain antineoplastic agents has been recommended. CPT-11-based
regimens, including FOLFIRI and IRIS, one of the major
chemotherapeutic regimens for colorectal cancer, are another
example. Our results demonstrated that the CR and TC rates in
CPT-11-based regimens were lower and the VAS scores were
significantly higher compared to the other MEC regimens (data not
shown). Thus, we concluded that it may be necessary to include
NK1RA as part of the three-drug combination
(5-HT3RA, NK1RA and dexamethasone), similar
to HEC. Additionally, there have been a number of cases in which
breakthrough emesis developed despite the patient being on a
three-drug antiemetic regimen, which is another major issue of CINV
that needs to be addressed in the immediate future. Although
existing guidelines recommend the use of domperidone and
metoclopramide as adjunct agents (5), the efficacy of these agents was not
found to be satisfactory. Olanzapine has relatively recently gained
recognition for its efficacy against hard-to-control CINV (22), becoming one of the promising
antiemetics. Furthermore, the use of Rikkunshito a traditional
Japanese (Kampo) medicine, previously shown to be efficient against
appetite loss in CDDP regimens (23), also needs to be taken into
consideration.
In conclusion, our data indicated that palonosetron
is effective and may be recommended as a 5-HT3RA, as it
is crucial that we take adequate measures against CINV to maintain
the patients’ quality of life and develop antiemetic regimens that
ensure the continuity of chemotherapy without dose reduction.
References
|
1.
|
Neymark N and Crott R: Impact of emesis on
clinical and economic outcomes of cancer therapy with highly
emetogenic chemotherapy regimens: a retrospective analysis of three
clinical trials. Support Care Cancer. 13:812–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
American Society of Clinical Oncology;
Kris Mg, Hesketh PJ, Somerfield MR, et al: American Society of
Clinical Oncology guideline for antiemetics in oncology: update
2006. J Clin Oncol. 24:2932–2947. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
National Comprehensive Cancer Network:
NCCN Practice Guidelines in Oncology. Available at:https://www.nccn.org. Accessed
August 10, 2013.
|
|
4.
|
Rolia F, Herrstedt J, Aapro M, et al:
ESMO/MASCC Guidelines Working Group: Guideline update for MASCC and
ESMO in the prevention of chemotherapy- and radiotherapy-induced
nausea and vomiting: results of the Perugia consensus conference.
Ann Oncol. 21:v232–v243. 2010. View Article : Google Scholar
|
|
5.
|
Japan Society of Clinical Oncology:
Guidelines for antiemetics in Oncology 2010. 1st edition. Japan:
Kanehara Publishing Company Ltd.; Tokyo, Japan:
|
|
6.
|
Tavorath R and Hesketh PJ: Drug treatment
of chemotherapy-induced delayed emesis. Drugs. 52:639–648. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hesket PJ: Chemotherapy-induced nausea and
vomiting. N Engl J Med. 358:2482–2494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
del Giglio A, Soares HP, Capparoz C and
Castro PC: Granisetron is equivalent to ondansetron for prophylaxis
of chemotherapy-induced nausea and vomiting: results of a
meta-analysis of randomized controlled trials. Cancer.
89:2301–2308. 2000.PubMed/NCBI
|
|
9.
|
Geling O and Eichler HG: Should
5-hydroxytryptamine-3 receptor antagonists be administered beyond
24 hours after chemotherapy to prevent delayed emesis? Systematic
re-evaluation of clinical evidence and drug cost implications. J
Clin Oncol. 23:1289–1294. 2005. View Article : Google Scholar
|
|
10.
|
Warr DG, Hesketh PJ, Gralla RJ, et al:
Efficacy and tolerability of aprepitant for the prevention of
chemotherapy-induced nausea and vomiting in patients with breast
cancer after moderately emetogenic chemotherapy. J Clin Oncol.
23:2822–2830. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Saito M, Aogi K, Sekine K, et al:
Palonosetron plus dexamethasone versus granisetron plus
dexamethasone for prevention of nausea and vomiting during
chemotherapy: a double-blind, double-dummy, randomised, comparative
phase III trial. Lancet Oncol. 10:115–124. 2009. View Article : Google Scholar
|
|
12.
|
Börjeson S, Hursti TJ, Peterson C, et al:
Similarities and differences in assessing nausea on a verbal
category scale and a visual analogue scale. Cancer Nurs.
20:260–266. 1997.PubMed/NCBI
|
|
13.
|
Del Favero A, Roila F, Basurto C, et al:
Assessment of nausea. Eur J Clin Pharmacol. 38:115–120. 1990.
|
|
14.
|
Maemondo M, Masuda N, Sekine I, et al: A
phase II study of palonosetron combined with dexamethasone to
prevent nausea and vomiting induced by highly emetogenic
chemotherapy. Ann Oncol. 20:1860–1866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Eisenberg P, Figueroa-Vadillo J, Zamora R,
et al 99-04 Palonosetron Study Group: Improved prevention of
moderately emetogenic chemotherapy-induced nausea and vomiting with
palonosetron, a pharmacologically novel 5-HT3 receptor
antagonist. Cancer. 98:2473–2482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ho CM, Wu HL, Ho ST and Wang JJ:
Dexamethasone prevents postoperative nausea and vomiting: benefit
versus risk. Acta Anaesthesiol Taiwan. 49:100–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
No authors listed: Double-blind,
dose-finding study of four intravenous doses of dexamethasone in
the prevention of cisplatin-induced acute emesis. Italian Group for
Antiemetic Research. J Clin Oncol. 16:2937–2942. 1998.PubMed/NCBI
|
|
18.
|
Grunberg SM: Antiemetic activity of
corticosteroids in patients receiving cancer chemotherapy: dosing,
efficacy, and tolerability analysis. Ann Oncol. 18:233–240. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tonato M, Clark-Snow RA, Osoba D, et al:
Emesis induced by low or minimal emetic risk chemotherapy. Support
Care Cancer. 13:109–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Italian Group for Antiemetic Research:
Dexamethasone, granisetron, or both for the prevention of nausea
and vomiting during chemotherapy for cancer. N Engl J Med. 332:1–5.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Roila F, Hesketh PJ, Herrstedt J, et al:
Prevention of chemotherapy- and radiotherapy-induced emesis:
results of the 2004 Perugia international antiemetic consensus
conference. Ann Oncol. 17:20–28. 2006.PubMed/NCBI
|
|
22.
|
Tan L, Liu J, Liu X, et al: Clinical
research of olanzapine for prevention of chemotherapy-induced
nausea and vomiting. J Exp Clin Cancer Res. 23:1312009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Takeda H, Sadakane C, Hattori T, et al:
Rikkunshito, an herbal medicine, suppresses cisplatin-induced
anorexia in rats via 5-HT2 receptor antagonism.
Gastroenterology. 134:2004–2013. 2008. View Article : Google Scholar : PubMed/NCBI
|