|
1.
|
Neymark N and Crott R: Impact of emesis on
clinical and economic outcomes of cancer therapy with highly
emetogenic chemotherapy regimens: a retrospective analysis of three
clinical trials. Support Care Cancer. 13:812–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
American Society of Clinical Oncology;
Kris Mg, Hesketh PJ, Somerfield MR, et al: American Society of
Clinical Oncology guideline for antiemetics in oncology: update
2006. J Clin Oncol. 24:2932–2947. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
National Comprehensive Cancer Network:
NCCN Practice Guidelines in Oncology. Available at:https://www.nccn.org. Accessed
August 10, 2013.
|
|
4.
|
Rolia F, Herrstedt J, Aapro M, et al:
ESMO/MASCC Guidelines Working Group: Guideline update for MASCC and
ESMO in the prevention of chemotherapy- and radiotherapy-induced
nausea and vomiting: results of the Perugia consensus conference.
Ann Oncol. 21:v232–v243. 2010. View Article : Google Scholar
|
|
5.
|
Japan Society of Clinical Oncology:
Guidelines for antiemetics in Oncology 2010. 1st edition. Japan:
Kanehara Publishing Company Ltd.; Tokyo, Japan:
|
|
6.
|
Tavorath R and Hesketh PJ: Drug treatment
of chemotherapy-induced delayed emesis. Drugs. 52:639–648. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hesket PJ: Chemotherapy-induced nausea and
vomiting. N Engl J Med. 358:2482–2494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
del Giglio A, Soares HP, Capparoz C and
Castro PC: Granisetron is equivalent to ondansetron for prophylaxis
of chemotherapy-induced nausea and vomiting: results of a
meta-analysis of randomized controlled trials. Cancer.
89:2301–2308. 2000.PubMed/NCBI
|
|
9.
|
Geling O and Eichler HG: Should
5-hydroxytryptamine-3 receptor antagonists be administered beyond
24 hours after chemotherapy to prevent delayed emesis? Systematic
re-evaluation of clinical evidence and drug cost implications. J
Clin Oncol. 23:1289–1294. 2005. View Article : Google Scholar
|
|
10.
|
Warr DG, Hesketh PJ, Gralla RJ, et al:
Efficacy and tolerability of aprepitant for the prevention of
chemotherapy-induced nausea and vomiting in patients with breast
cancer after moderately emetogenic chemotherapy. J Clin Oncol.
23:2822–2830. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Saito M, Aogi K, Sekine K, et al:
Palonosetron plus dexamethasone versus granisetron plus
dexamethasone for prevention of nausea and vomiting during
chemotherapy: a double-blind, double-dummy, randomised, comparative
phase III trial. Lancet Oncol. 10:115–124. 2009. View Article : Google Scholar
|
|
12.
|
Börjeson S, Hursti TJ, Peterson C, et al:
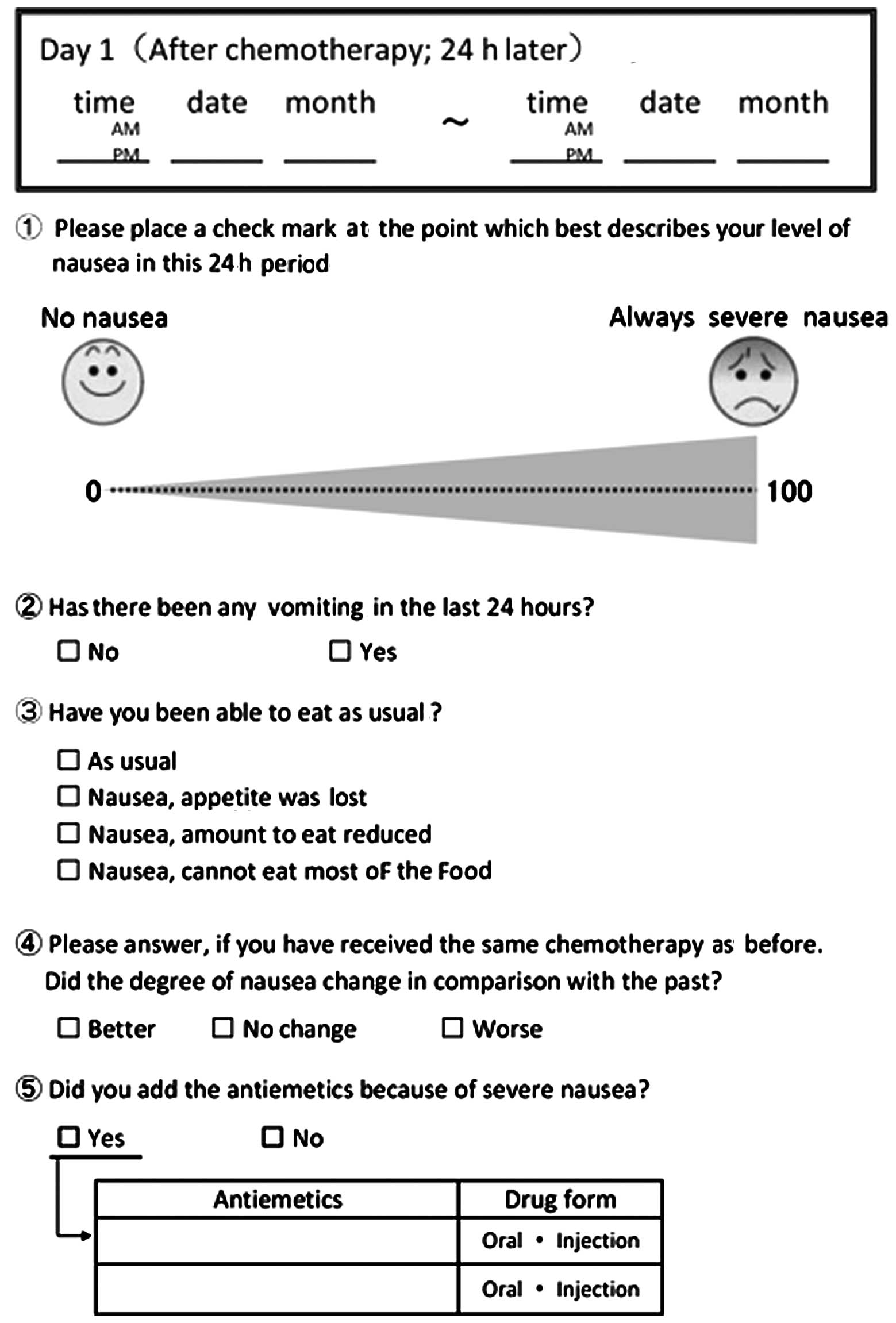
Similarities and differences in assessing nausea on a verbal
category scale and a visual analogue scale. Cancer Nurs.
20:260–266. 1997.PubMed/NCBI
|
|
13.
|
Del Favero A, Roila F, Basurto C, et al:
Assessment of nausea. Eur J Clin Pharmacol. 38:115–120. 1990.
|
|
14.
|
Maemondo M, Masuda N, Sekine I, et al: A
phase II study of palonosetron combined with dexamethasone to
prevent nausea and vomiting induced by highly emetogenic
chemotherapy. Ann Oncol. 20:1860–1866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Eisenberg P, Figueroa-Vadillo J, Zamora R,
et al 99-04 Palonosetron Study Group: Improved prevention of
moderately emetogenic chemotherapy-induced nausea and vomiting with
palonosetron, a pharmacologically novel 5-HT3 receptor
antagonist. Cancer. 98:2473–2482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ho CM, Wu HL, Ho ST and Wang JJ:
Dexamethasone prevents postoperative nausea and vomiting: benefit
versus risk. Acta Anaesthesiol Taiwan. 49:100–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
No authors listed: Double-blind,
dose-finding study of four intravenous doses of dexamethasone in
the prevention of cisplatin-induced acute emesis. Italian Group for
Antiemetic Research. J Clin Oncol. 16:2937–2942. 1998.PubMed/NCBI
|
|
18.
|
Grunberg SM: Antiemetic activity of
corticosteroids in patients receiving cancer chemotherapy: dosing,
efficacy, and tolerability analysis. Ann Oncol. 18:233–240. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tonato M, Clark-Snow RA, Osoba D, et al:
Emesis induced by low or minimal emetic risk chemotherapy. Support
Care Cancer. 13:109–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Italian Group for Antiemetic Research:
Dexamethasone, granisetron, or both for the prevention of nausea
and vomiting during chemotherapy for cancer. N Engl J Med. 332:1–5.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Roila F, Hesketh PJ, Herrstedt J, et al:
Prevention of chemotherapy- and radiotherapy-induced emesis:
results of the 2004 Perugia international antiemetic consensus
conference. Ann Oncol. 17:20–28. 2006.PubMed/NCBI
|
|
22.
|
Tan L, Liu J, Liu X, et al: Clinical
research of olanzapine for prevention of chemotherapy-induced
nausea and vomiting. J Exp Clin Cancer Res. 23:1312009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Takeda H, Sadakane C, Hattori T, et al:
Rikkunshito, an herbal medicine, suppresses cisplatin-induced
anorexia in rats via 5-HT2 receptor antagonism.
Gastroenterology. 134:2004–2013. 2008. View Article : Google Scholar : PubMed/NCBI
|