Introduction
Lung cancer, including small-cell lung cancer (SCLC)
and non-small-cell lung cancer (NSCLC), is the most common type of
cancer worldwide (1). Cisplatin
[cis-diammineplatinum dichloride (DDP)]-based chemotherapy
has long been used as first-line treatment for patients with SCLC
and advanced NSCLC (2–3). However, DDP resistance and tumor
relapse, which are likely mediated by cancer stem-like cells (CSCs)
or side-population (SP) cells, invariably occur (4–6).
CSCs or SP cells express high levels of breast
cancer resistance protein (ABCG2), which acts as an adenosine
triphosphate-dependent membrane transporter. ABCG2 efficiently
effluxes a variety of chemotherapeutic agents, such as DDP, in
chemoresistant cancer cells and may be the main mechanism
underlying resistance to chemotherapy (5,7–8).
Consistently, previous studies by Kim et al
(9) and Tang et al
(10) reported that decreased
platinum accumulation in NSCLC tumor tissues and SCLC cells may be
an important mechanism underlying platinum resistance in NSCLC and
SCLC cells in the clinical setting.
Over the last 20 years, high concentrations of
ethanol (>95%) alone have been successfully used for the
ablation of various types of tumors, particularly liver tumors
sized <3 cm (11–12). Although high concentrations of
ethanol may destroy various types of smaller tumors through
inducing tumor cell dehydration and necrosis, protein degeneration
in larger tumors may result in boundary formation, which causes
some areas to be left unharmed (11–12).
Moreover, Tan et al (13)
and Forsyth et al (14)
demonstrated that the administration of ethanol alone may stimulate
angiogenesis and promote tumor growth.
We previously demonstrated that a low concentration
of ethanol (5%) was able to potently inhibit the ABCG2 pump in lung
CSCs (15). The inhibition of
ABCG2 results in the deposition of cytotoxic DDP into lung CSCs and
other cancer cells, efficiently eliminating them (15–16).
DDP in 5% ethanol (5% ethanol-DDP) injected intratumorally was able
to eradicate DDP-resistant lung tumors and extend survival by
eliminating lung CSCs and non-CSC cancer cells in our previous
xenograft study (15).
However, there may be an optimal concentration of
ethanol in which DDP exhibits its highest efficacy in reducing
tumor volume and extending survival compared to 5% ethanol-DDP.
Therefore, the present study aimed to compare the
efficiency of DDP in 2, 10, 20 and 50% ethanol to that of 5%
ethanol-DDP for the treatment of DDP-resistant lung tumor-bearing
nude mice. In addition, we investigated the potential mechanisms
underlying the differences in DDP efficiency by
immunohistochemistry (IHC) analysis of microvessel density (MVD) in
xenograft tumor tissues.
Materials and methods
Ethics statement
All animal experiments were conducted with the
approval of the Institutional Animal Care and Use Committee of the
China Agricultural University (Haidian, China). The conditions of
the animals were monitored daily for evidence of illness. Four
steps were taken to minimize the suffering of the animals as
follows: i) air exchange, temperature, humidity, noise, light
intensity and light cycles were maintained within limits compatible
with the health and well-being of the mice; ii) cleaning practices
were monitored on a regular basis to ensure effective hygiene and
sterile sanitation; iii) to avoid unnecessary harm, the agents were
injected gently; and iv) mice showing severe distress, including
infection, ulceration, cachexia, inability to ambulate and
moribund, were humanely euthanized by cervical dislocation in
accordance with animal care protocols. All the mice were euthanized
after 180 days of observation. Two mice that exhibited severe
distress due to biting infection in the tumors succumbed to
non-tumor-related factors.
A number of mice in this study developed sizeable
tumors, as survival research requires as long an observation time
as possible. Moreover, we previously reported that mice receiving
ethanol treatment developed larger tumors and better general
condition compared to control mice. As a result, mice with sizeable
tumors appeared to be in a good overall condition even up to 3
weeks prior to the onset of severe distress.
Cancer cell culture and reagents
The DDP-resistant A549/DDP human lung adenocarcinoma
cell line was obtained from the American Type Culture Collection
(Rockville, MD, USA) and was routinely cultured in RPMI medium
supplemented with 10% fetal bovine serum (Invitrogen China Ltd.,
Beijing, China) and 2 μmol DDP (Qilu Pharmaceutical Co., Ltd.,
Jinan, China). The A549/DDP cells were cultured in complete RPMI
medium without DDP for 3 days prior to use. Ethanol (>99.9%)
(v/v) was purchased from Sinopharm Chemical Reagent Co. (Beijing,
China). Concentrations of 2, 5, 10, 20 and 50% ethanol (v/v) were
prepared with >99.9% ethanol and sterile water. Concentrations
of 2, 5, 10, 20 and 50% ethanol-DDP were freshly prepared with 8
mg/kg DDP (Qilu Pharmaceutical Co., Ltd.) dissolved in a
corresponding concentration of ethanol solution of 150 μl.
Tumor growth xenograft study
A total of 180 inbred male Balb/C nude mice, aged 6
weeks, were obtained from the Institute of the Chinese Association
for Laboratory Animal Sciences (Beijing, China). A549/DDP lung
adenocarcinoma cells (5×106) were subcutaneously
inoculated in the upper left flank on day 1. When the tumor
diameters had exceeded 5 mm, the mice were divided into the control
group, the 2, 5, 10, 20 and 50% ethanol groups, the DDP group and
the 2, 5, 10, 20 and 50% ethanol + DDP groups (n=15 per group). The
mice in each group were treated with intratumoral injections of 150
μl sterile water (control); 2, 5, 10, 20 or 50% ethanol; 8 mg/kg
DDP in sterile water; or 2, 5, 10, 20 or 50% ethanol + 8 mg/kg DDP,
accordingly, twice a week for 4 weeks. Tumor volumes were measured
with calipers twice a week and calculated using the following
equation: (width2 × length)/2 (5). The survival of mice in each group was
observed and recorded. To minimize the suffering of the animals,
cleaning practices were monitored on a regular basis to ensure
effective hygiene and sterile sanitation. To avoid unnecessary
harm, the agents were injected gently. The condition of the animals
was monitored daily for evidence of illness. Pain reaction
associated with intratumoral injection was also observed. Mice
showing severe distress, including infection, ulceration, cachexia,
inability to ambulate and moribund were euthanized by cervical
dislocation in accordance with animal care protocols. All the mice
were euthanized after 180 days of observation.
ICH staining of CD34 in tumor
tissues
The paraffin-embedded tissues were cut into 4-μm
sections and heated at 60°C for 60 min. The sections were
deparaffinized with xylene and rehydrated, then treated with 3%
hydrogen peroxide in methanol, followed by incubation with normal
serum to block non-specific binding. The slides were incubated with
anti-mouse CD34 antibody (Abcam, Cambridge, UK) at 4°C overnight.
After washing, the tissue sections were treated with anti-rat
secondary antibody (Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China), followed by further incubation with
horseradish peroxidase-labelled polymer (Dako, Carpinteria, CA,
USA) for 20 min. Following staining with diaminobenzidine
(Sigma-Aldrich, Munich, Germany), the sections were counterstained
with hematoxylin (Sigma-Aldrich). The known positive tissues were
used as positive controls and the primary antibody was omitted for
negative controls. The staining of targeted proteins was visualized
under an Olympus microscope (Olympus, Tokyo, Japan).
The CD34 antibody-stained slides were reviewed and
scored independently by two observers. MVD was detected using
immunostaining, as previously described by Weidner (17). The mean score of 5 fields was
considered to be the level of MVD for each sample.
Statistical analysis
Data are expressed as means ± standard deviation
except in the analysis of survival data, which are expressed as
means ± standard error of the mean. Paired and unpaired Student’s
t-tests were used to analyze two groups of paired or unpaired data,
respectively. Repeated measured analysis of variance was used for
the comparison of multiple groups. Survival analysis was performed
according to the Kaplan-Meier method and the log-rank (Mantel-Cox)
test using SPSS statistical software version 19.0 (IBM, Armonk, NY,
USA). Mice that remained alive at the end of the study were
censored. P<0.05 was considered to indicate a statistically
significant difference.
Results
Tumor size changes in different groups of
DDP-resistant lung tumor-bearing mice
After 4 weeks of treatment, the mean tumor volume in
the 5% ethanol-DDP group (0.11±0.06 cm3) was
statistically lower compared to that in the 2, 10, 20 or 50%
ethanol-DDP groups (0.22±0.08 cm3, 0.78±0.36
cm3, 1.36±0.53 cm3 and 1.43±0.71
cm3, respectively; P<0.01) (Fig. 1A). Administration of 5% ethanol-DDP
to A549/DDP tumor-bearing mice resulted in complete tumor
regression in 3 out of 10 mice (30%) and tumor growth arrest in the
remaining 7 mice (70%) after 4 weeks of treatment. By contrast,
only 1 out of 10 mice (10%) treated with 2% ethanol-DDP and no mice
in the other groups achieved complete tumor regression. Compared to
the tumor size in control mice (3.68±0.48 cm3),
treatment with 2, 5, 10, 20 or 50% ethanol alone was shown to
stimulate tumor growth (4.31±0.12 cm3, P<0.01;
4.23±0.63 cm3, P<0.05; 4.32±0.63 cm3,
P<0.01; 4.59±0.36 cm3, P<0.01; and 4.67±0.26
cm3, P<0.01, respectively) (Fig. 1A).
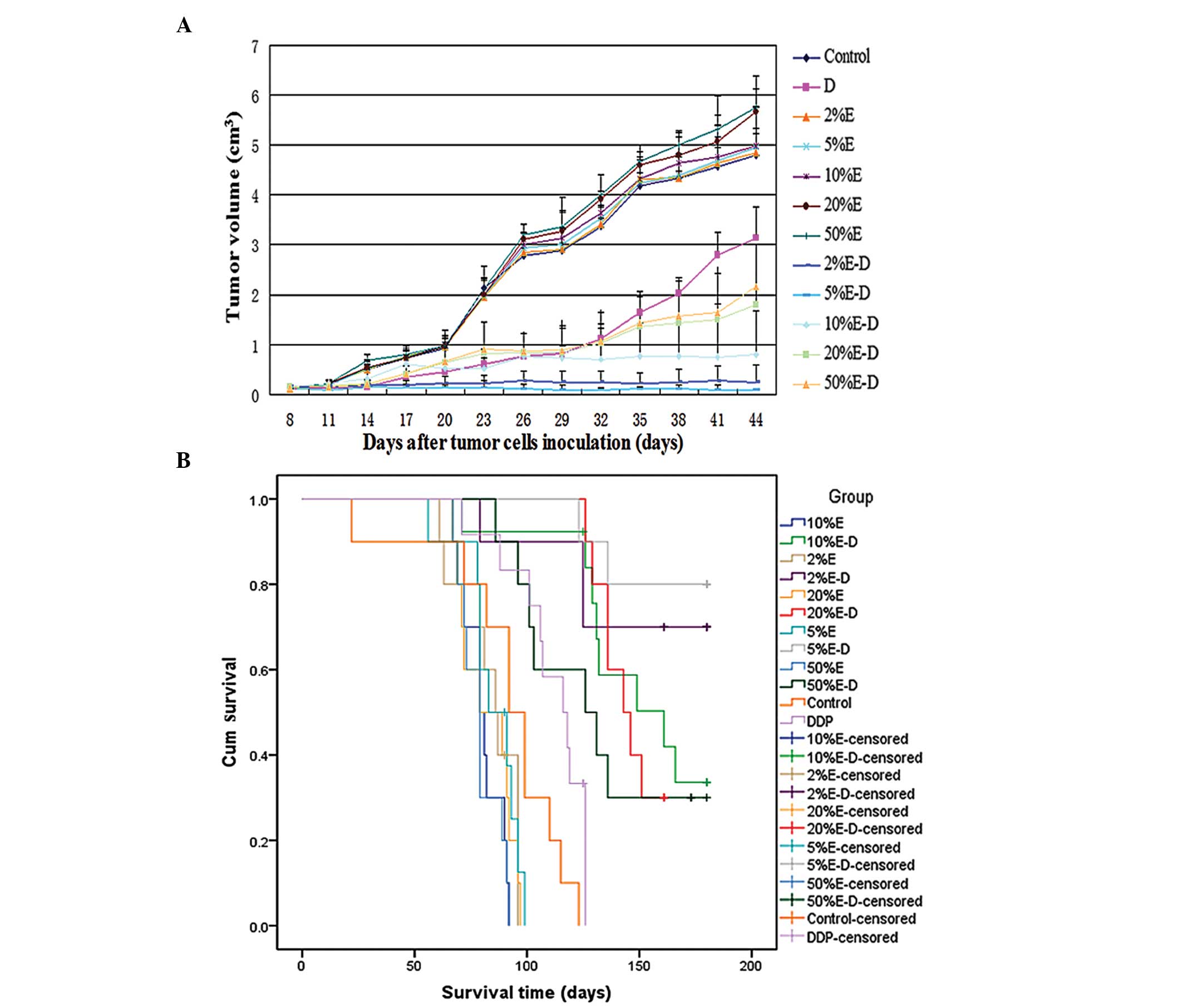 | Figure 1Changes in tumor size and survival in
DDP-resistant lung tumor-bearing Balb/C mice following treatment
with DDP in various concentrations of ethanol. (A) Changes in tumor
size following various treatments. After 4 weeks of treatment, the
mean tumor volume in mice treated with 5% ethanol-DDP (0.11±0.06
cm3) was statistically lower compared to that in mice
treated with 2, 10, 20 and 50% ethanol-DDP (0.22±0.08, 0.78±0.36,
1.36±0.53 and 1.43±0.71 cm3, respectively; P<0.01).
Treatment with 5% ethanol-DDP was the most effective in reducing
tumor volumes in DDP-resistant tumor-bearing mice. (B) Survival of
A549/DDP tumor-bearing mice following various treatments. Treatment
with 5% ethanol-DDP achieved the longest estimated mean survival
among all the treatment groups. The estimated mean survival time in
5% ethanol-DDP-treated mice (169.9±6.5 days) was significantly
longer compared to that in mice treated with 10, 20 and 50%
ethanol-DDP (149.3±8.8, 145.0±4.0 and 131.9±11.0 days,
respectively; P<0.05). Although there were no significant
differences between 2 and 5% ethanol-DDP-treated mice, the
estimated mean survival time in the 5% ethanol-DDP mice tended to
be longer compared to that in the 2% ethanol-DDP-treated mice
(169.9±6.5 vs. 158.9±10.9 days; P>0.05). E, ethanol; D/DDP,
cisplatin; cum, cumulative. |
Survival of tumor-bearing nude mice in
different groups
Treatment with 5% ethanol-DDP achieved the longest
survival among all the investigated treatment groups. After 180
days of observation, 80% (8 out of 10) of the 5%
ethanol-DDP-treated mice remained alive, with two deaths from
non-tumor-related reasons; all 10 control mice died prior to day
123.
The mean survival of the 5% ethanol-DDP-treated
group (169.9±6.5 days) was found to be significantly longer
compared to that of the 10, 20 and 50% ethanol-DDP, DDP, control
and 2, 5 10, 20 and 50% ethanol alone groups (149.3±8.8 days,
P<0.05; 145.0±4.0 days, P<0.05; 131.9±11.0 days, P<0.05;
110.8±5.1 days, P<0.01; 90.6±9.0 days, P<0.01; 84.1±4.3 days,
P<0.01; 84.9±4.1 days, P<0.01; 80.2±2.8 days, P<0.01;
82.3±3.8 days for 20%, P<0.01; and 79.0±2.9 days, P<0.01,
respectively). Although there were no significant differences
between 2 and 5% ethanol-DDP-treated mice, the estimated mean
survival time of 5% ethanol-DDP-treated mice tended to be longer
compared to that of the 2% ethanol-DDP-treated mice (169.9±6.5 vs.
158.9±10.9 days, respectively; P>0.05) (Fig. 1B).
Effects of various combinations of
ethanol-DDP on tumor angiogenesis by IHC analysis of MVD
Compared to the MVD of 30.2±3.5 in the control
group, treatment with 2, 5 and 10% ethanol-DDP significantly
decreased MVD in the tumor tissues (21.3±3.6, P<0.01; 15.3±3.1,
P<0.01; and 23.6±3.1, P<0.05, respectively). Among these
combinations, 5% ethanol-DDP produced the most significant MVD
reduction compared to that in the control group (Fig. 2). MVD in tumor tissues treated with
20 and 50% ethanol-DDP was not significantly altered compared to
the MVD of the control group (26.5±5.3 and 33.3±5.6, respectively;
P>0.05). Compared to a MVD of 30.2±3.5 in the control group,
various concentrations of ethanol alone increased MVD (42.2±5.8 for
2%, 50.7±5.3 for 5%, 57.4±6.1 for 10%, 61.7±7.3 for 20% and
65.3±6.1 for 50% ethanol; P<0.01). In addition to the
stimulation of tumor growth, ethanol at higher concentrations, such
as 20 and 50%, also caused local injection pain.
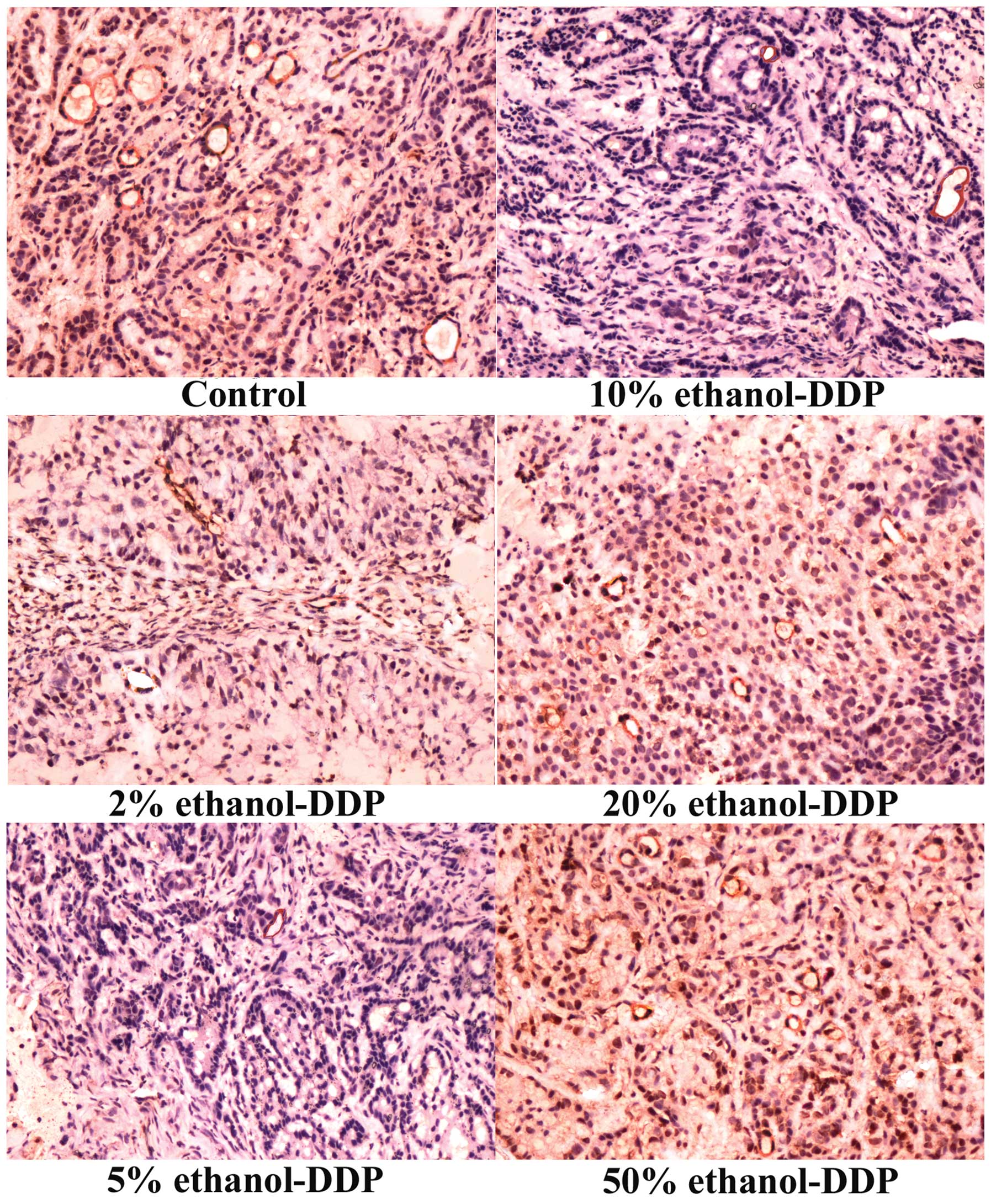 | Figure 2Immunohistochemistry analysis of
microvessel density (MVD) in tumor tissues. MVD staining of tumor
tissues in control (upper left panel), 2% (middle left panel), 5%
(bottom left panel), 10% (upper right panel), 20% (middle right
panel) and 50% ethanol-DDP-treated mice (bottom right panel).
Compared to the MVD of 30.2±3.5 in control tumor tissues, treatment
with 2, 5 and 10% ethanol-DDP significantly decreased MVD in tumor
tissues (21.3±3.6, P<0.01; 15.3±3.1, P<0.01; and 23.6±3.1,
P<0.05, respectively). Among these, 5% ethanol-DDP produced the
most significant MVD reduction compared to that in the control
samples. The MVD in the tumor tissues of mice treated with 20 and
50% ethanol-DDP was not significantly altered compared to the MVD
of the control tissues (26.5±5.3 and 33.3±5.6; P>0.05,
respectively). DDP, cisplatin. |
Discussion
Our study demonstrated that treatment with 5%
ethanol-DDP achieved the highest efficiency among all the
investigated ethanol-DDP combinations in reducing tumor volume and
prolonging survival. The results of the MVD analysis suggested that
the potent inhibition of tumor angiogenesis by 5% ethanol-DDP may
be one of the key mechanisms underlying its superior
efficiency.
Treatment with 5% ethanol-DDP achieved the most
significant tumor growth inhibition compared to 2, 5, 10, 20 and
50% ethanol-DDP. Treatment with 5% ethanol-DDP also induced
complete tumor regression in 3 out of 10 mice (30%) and tumor
growth arrest in the remaining 7 mice (70%) after 4 weeks of
treatment. By contrast, only 1 of the 10 mice (10%) treated with 2%
ethanol-DDP and no mice in the other groups achieved complete tumor
regression.
In addition, 5% ethanol-DDP treatment produced the
longest survival among all groups of mice. After 180 days of
observation, 80% (8 out of 10) of the 5% ethanol-DDP-treated mice
remained alive, with two deaths from non-tumor-related reasons.
Mechanistically, 5% ethanol-DDP exhibited the most
significant tumor angiogenesis inhibition among all the
investigated ethanol-DDP combinations, whereas various
concentrations of ethanol alone were shown to increase tumor
angiogenesis. Our results suggested that DDP inhibited tumor
angiogenesis most potently in 5% ethanol and this may be one of the
mechanisms underlying its superior efficiency.
Previous studies by Pietronigro et al
(18) and Jenkinson et al
(19) reported that intratumoral
injection of the chemotherapeutic agent carmustine dissolved in
100% ethanol, achieved a 40% cure rate in rats bearing intracranial
T9 tumors and 72% stable disease in patients with recurrent
malignant glioma. However, our results demonstrated that DDP, when
dissolved in high concentrations of ethanol, such as 50% ethanol,
exhibited minimal tumor inhibition with obvious normal tissue
damage compared to DDP dissolved in lower ethanol concentrations.
We also observed that higher concentrations of ethanol
significantly promoted tumor growth, which was associated with
increased tumor angiogenesis. These results were consistent with
those of previous studies by Tan et al (13) and Forsyth et al (14), suggesting that ethanol alone
stimulates angiogenesis and promotes tumor growth. A possible
explanation for the differences between our results and those of
previous studies may be that the glioma tumors in the rats in those
studies were smaller compared to the tumors in our study. Smaller
tumors may be easier to diffuse and sufficiently suffuse by 100%
ethanol, resulting in complete tumor necrosis. By contrast, the
tumors in our studies were difficult to evenly diffuse due to their
large size and required higher concentrations of ethanol, leaving
some tumor cells unharmed and activating tumor angiogenesis.
In our previous xenograft study, we demonstrated
that 5% ethanol-DDP injected intratumorally was able to eradicate
DDP-resistant lung tumors and prolong survival by eliminating lung
CSCs and non-CSC cancer cells (15). However, whether there was an
optimal ethanol concentration in which DDP achieved a higher
efficacy compared to that of 5% ethanol-DDP had not been
determined. The potential mechanisms underlying the efficiency of
DDP in different concentrations of ethanol had not been fully
elucidated. The present study established that 5% ethanol-DDP was
the optimal combination compared to that of DDP with 2, 5, 10, 20
and 50% ethanol in controlling DDP-resistant lung tumors. We also
found that 5% ethanol-DDP inhibited tumor angiogenesis most
significantly compared to other combinations and may be one of the
mechanisms underlying its superior efficiency.
This study confirmed 5% ethanol-DDP as the optimal
combination for the treatment of DDP-resistant lung tumors among
the combinations of DDP with 2, 5, 10, 20 and 50% ethanol. Ethanol
and DDP have been used safely in the clinical setting over several
decades; however, the application of this combination to improve
the prognosis of DDP-resistant lung cancer warrants further
investigation.
In conclusion, our results demonstrated that 5%
ethanol-DDP achieved the most potent tumor growth inhibition and
prolongation of survival compared to the other investigated
ethanol-DDP combinations, providing an effective method to improve
the survival of patients with DDP-resistant lung cancer. However,
further clinical studies are required to clearly determine the
efficacy and safety of this novel approach.
Acknowledgements
The authors would like to thank Dr Li Li
(Tuberculosis Research Institute, 309 PLA Hospital) for his
assistance and valuable advice in the animal assay. This study was
supported by the Scientific Research Foundation for the Returned
Overseas Chinese Scholars, China State Education Ministry (grant
no. 2007-1108).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Rossi A, Di Maio M, Chiodini P, et al:
Carboplatin- or cisplatin-based chemotherapy in first-line
treatment of small-cell lung cancer: the COCIS meta-analysis of
individual patient data. J Clin Oncol. 30:1692–1698. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ardizzoni A, Boni L, Tiseo M, et al; CISCA
(CISplatin versus CArboplatin) Meta-analysis Group. Cisplatin-
versus carboplatin-based chemotherapy in first-line treatment of
advanced non-small-cell lung cancer: an individual patient data
meta-analysis. J Natl Cancer Inst. 99:847–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eramo A, Haas TL and De Maria R: Lung
cancer stem cells: tools and targets to fight lung cancer.
Oncogene. 29:4625–4635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niu Q, Wang W, Li Y, Ruden DM, Wang F, Li
Y, Wang F, Song J and Zheng K: Low molecular weight heparin ablates
lung cancer cisplatin-resistance by inducing proteasome-mediated
ABCG2 protein degradation. PLoS One. 7:e410352012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
7
|
Singh A, Wu H, Zhang P, Happel C, Ma J and
Biswal S: Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer
cells that confers side population and chemoresistance phenotype.
Mol Cancer Ther. 9:2365–2376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang M, Mathur A, Zhang Y, et al:
Mithramycin represses basal and cigarette smoke-induced expression
of ABCG2 and inhibits stem cell signaling in lung and esophageal
cancer cells. Cancer Res. 72:4178–4192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim ES, Lee JJ, He G, Chow CW, Fujimoto J,
Kalhor N, Swisher SG, Wistuba II, Stewart DJ and Siddik ZH: Tissue
platinum concentration and tumor response in non-small-cell lung
cancer. J Clin Oncol. 30:3345–3352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang CH, Parham C, Shocron E, McMahon G
and Patel N: Picoplatin overcomes resistance to cell toxicity in
small-cell lung cancer cells previously treated with cisplatin and
carboplatin. Cancer Chemother Pharmacol. 67:1389–1400. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livraghi T, Festi D, Monti F, Salmi A and
Vettori C: US-guided percutaneous alcohol injection of small
hepatic and abdominal tumors. Radiology. 161:309–312. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuang M, Lu MD, Xie XY, Xu HX, Xu ZF, Liu
GJ, Yin XY, Huang JF and Lencioni R: Ethanol ablation of
hepatocellular carcinoma up to 5.0 cm by using a multipronged
injection needle with high-dose strategy. Radiology. 253:552–561.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan W, Bailey AP, Shparago M, Busby B,
Covington J, Johnson JW, Young E and Gu JW: Chronic alcohol
consumption stimulates VEGF expression, tumor angiogenesis and
progression of melanoma in mice. Cancer Biol Ther. 6:1211–1217.
2007.PubMed/NCBI
|
|
14
|
Forsyth CB, Tang Y, Shaikh M, Zhang L and
Keshavarzian A: Alcohol stimulates activation of Snail, epidermal
growth factor receptor signaling, and biomarkers of
epithelial-mesenchymal transition in colon and breast cancer cells.
Alcohol Clin Exp Res. 34:19–31. 2010. View Article : Google Scholar
|
|
15
|
Niu Q, Wang W, Li Y, Ruden DM, Li Q and
Wang F: Cisplatin in 5% ethanol eradicates cisplatin-resistant lung
tumor by killing lung cancer side population (SP) cells and non-SP
cells. Front Genet. 4:1632013.
|
|
16
|
Hamstra DA, Moffat BA, Hall DE, Young JM,
Desmond TJ, Carter J, Pietronigro D, Frey KA, Rehemtulla A and Ross
BD: Intratumoral injection of BCNU in ethanol (DTI-015) results in
enhanced delivery to tumor - a pharmacokinetic study. J Neurooncol.
73:225–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pietronigro D, Drnovsky F, Cravioto H and
Ransohoff J: DTI-015 produces cures in T9 gliosarcoma. Neoplasia.
5:17–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jenkinson MD, Smith TS, Haylock B, Husband
D, Shenoy A, Vinjamuri S, Walker C, Pietronigro D and Warnke PC:
Phase II trial of intratumoral BCNU injection and radiotherapy on
untreated adult malignant glioma. J Neurooncol. 99:103–113. 2010.
View Article : Google Scholar : PubMed/NCBI
|
















