Introduction
With the advances in the multidisciplinary treatment
of pancreatic cancer (PC) over the last few years, it is crucial to
obtain a histopathological diagnosis prior to treatment. With the
introduction of endoscopic retrograde cholangiopancreatography
(ERCP), ERCP-guided pancreatic juice and bile cytology and bile and
pancreatic duct brush cytology or biopsy were adopted for the
diagnosis of PC (1,2). Vilmann et al (3) subsequently developed endoscopic
ultrasound-guided fine-needle aspiration (EUS-FNA), expanding the
range of endoscopic collection methods for PC. As ERCP requires a
highly skilled technique and, particularly, due to the risk of
post-ERCP pancreatitis (PEP), EUS-FNA, which is considered to have
a low incidence of complications, was widely adopted, primarily in
Western countries. However, the use of EUS-FNA in Japan has been
delayed, due to concerns regarding cancer seeding associated with
EUS-FNA (4); therefore, the number
of institutions performing EUS-FNA for pancreatic cystic lesions or
PC that have been scheduled for resection is currently limited.
Consequently, in Japan, endoscopic cytology or histological
diagnosis for PC is currently performed with a combination of ERCP
and EUS-FNA; however, the number of available studies is
insufficient to clearly determine the optimal endoscopic collection
method.
The purpose of this clinical study was to
retrospectively evaluate the results of endoscopic cytology
diagnosis in unresectable PC by the conventionally performed
ERCP-guided collection method and after the introduction of EUS-FNA
and to determine the usefulness of the two methods and the
associated complications.
Patients and methods
Patients
A total of 263 consecutive patients with
unresectable PC who underwent endoscopic cytology in our hospital
between 2002 and 2012 were included in this study. Unresectability
was confirmed in cases where a surgeon and a radiologist performed
various diagnostic imaging examinations and diagnosing the case as
Japan Pancreas Society classification stage 4a (major blood vessel
invasion) or stage 4b (distant metastases), cases in which surgery
was not feasible due to the risk of general anesthesia because of
cardiopulmonary disease and cases in which the patient was elderly
and was considered to be unlikely to tolerate surgery. PC was
definitively diagnosed based on a 6-month or longer course with
radiological imaging, clinical symptoms and biochemical
findings.
ERCP-guided pancreato-biliary brush
cytology and EUS-guided fine-needle aspiration
Prior to 2006, the endoscopic cell or tissue
collection method for unresectable PCs in our hospital consisted of
performing ERCP-guided pancreatic juice cytology (including
pancreatic duct brush cytology) or bile duct cytology as the first
choice. Since 2007, when EUS-FNA was introduced, ERCP-guided
specimen collection is adopted as the first choice when endoscopic
biliary stenting (EBS) is performed to relieve obstructive jaundice
and EUS-FNA is adopted as the first choice (EUS-FNA 1st) in cases
without obstructive jaundice. Additionally, in cases where an
adequate specimen was not obtained by ERCP-guided collection,
EUS-FNA may be subsequently performed (EUS-FNA 2nd) after obtaining
the patient’s consent.
In the ERCP-guided collection method, a guidewire
was inserted into the stricture of the pancreatic or bile duct and
the stricture was brushed 5–10 times with a brush catheter (Rapid
Cytology Brush, Boston Scientific, Tokyo, Japan; BC-24Q Disposable
Cytology Brush, Olympus, Tokyo, Japan; or Fusion Cytology Brush,
Cook Medical, Winston-Salem, NC, USA) that was inserted with the
ropeway method. After brushing, only the inner tube of the brush
catheter was removed and bile or pancreatic juice was collected by
aspiration in the pancreatic or bile duct proximal and distal to
the stricture. Only the tip of the brush catheter was removed and,
following immersion in physiological saline or a preservative
solution (Cyto-rich® Red Preservative,
Beckton-Dickinson, Franklin Lanes, NJ, USA), it was manually
stirred and the pellet obtained by centrifugation (840 × g × 5 min)
was used to prepare the slide specimens. The bile or pancreatic
fluid after brushing was also centrifuged in a similar manner and
slide specimens were prepared.
EUS-FNA specimen collection was performed mainly
using a 22G/25G puncture needle; 15–20 strokes and 3–5 sessions
were performed. The specimen was fixed in formalin and submitted
for cytology. Rapid on-site evaluation (ROSE) was also performed in
each case.
Patient grouping
The subjects were divided into two groups, one prior
to 2006 (group A), when the ERCP-guided collection method was
considered the first choice, and one from 2007 onwards (group B),
after the introduction of EUS-FNA. We compared the results of
cytology and histological diagnosis in the two groups and according
to the lesion site (pancreatic head vs. body and tail) and assessed
the incidence and nature of complications.
When the results of cytology or histological
diagnosis were ‘malignant’ or ‘suspected malignant’ the results
were considered to be cancer-positive. In addition, in cases where
ERCP-guided bile and pancreatic duct cytology was performed,
specimens of either method that were positive were considered to be
cancer-positive.
Statistical analysis
The JMP® 10 software program (SAS
Institute Inc., Cary, NC, USA) was used to perform the statistical
analysis and a P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The 263 PC cases that were diagnosed as unresectable
included 101 cases in group A and 162 in group B. The differences
between the groups regarding age, gender, tumor location, presence
or absence of obstructive jaundice and stage are summarized in
Table I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Group A | Group B | P-value |
|---|
| No. of patients | 101 | 162 | |
| Age, years mean
(range) | 68.91 (35–91) | 68.24 (33–90) | 0.631 |
| Gender,
male/female | 59/42 | 94/68 | 0.950 |
| Location of the
pancreatic tumor | | | |
| Head (presence of
obstructive jaundice) | 59 (39) | 74 (50) | |
| Body and tail | 42 | 88 | |
| Stage (JPS) | | | |
| 1 | 0 | 0 | 0.306 |
| 2 | 0 | 3 | |
| 3 | 8 | 9 | |
| 4a | 36 | 54 | |
| 4b | 57 | 96 | |
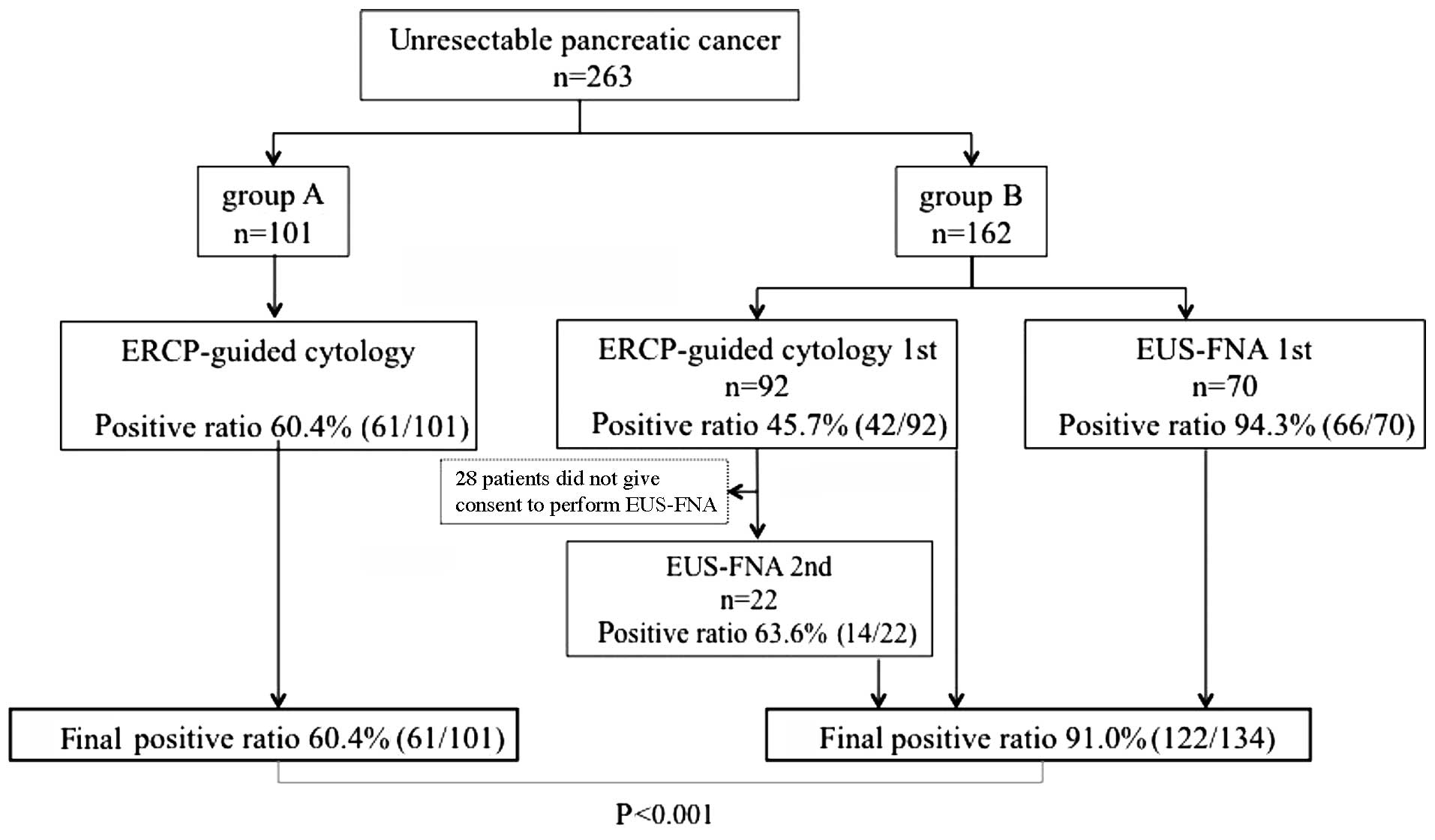
Comparison of endoscopic cytology results
between groups A and B
ERCP-guided cytology was performed in all 101
patients in group A, with a cancer-positive rate of 60.4% (61/101).
The 162 patients in group B included 92 patients in the ERCP-guided
cytology 1st group and 70 patients in the EUS-FNA 1st group. The
cancer-positive rate was 45.7% (42/92) in the ERCP-guided cytology
1st group and 94.3% (66/70) in the EUS-FNA 1st group. Consent to
subsequently perform EUS-FNA was obtained from 22 of the 50
patients in the ERCP-guided cytology 1st group who were
cancer-negative and the cancer-positive rate was 63.6% (14/22).
Collectively, the cancer-positive rate was 60.4% (61/101) in group
A and 75.3% (122/162) in group B; the difference was statistically
significant (P=0.01). Furthermore, when the 28 patients in group B
who did not consent to EUS-FNA after ERCP were excluded, the
cancer-positive rate in group B group was 91.0% (122/134), which
was significantly higher compared to that in group A (P<0.001)
(Fig. 1).
Endoscopic cytology results by
location
The cancer-positive rate of the lesions located in
the pancreatic head was 61.0% (36/59) in group A and 67.9% (57/84)
in group B; the difference was not statistically significant. The
cancer-positive rate in the pancreatic body and tail was 59.5%
(25/42) in group A and 83.3% (65/78) in group B and the difference
was statistically significant (P=0.005). The cancer-positive rate
of the EUS-FNA cases alone in group B was 75.7% (25/33) in the
pancreatic head and 93.2% (55/59) in the pancreatic body and tail,
with a statistically significant difference (P=0.017) (Table II).
 | Table IIComparison of endoscopic cytology
results according to the location of the pancreatic cancer between
groups A and B. |
Table II
Comparison of endoscopic cytology
results according to the location of the pancreatic cancer between
groups A and B.
| Location of
pancreatic cancer | Group A | Group B | P-value |
|---|
| Head | 61.0% (36/59) | 67.9% (57/84) | 0.134 |
| | ERCP 1st: 48.5%
(32/66 | |
| | EUS-FNA 1st: 88.9%
(16/18) | |
| | EUS-FNA 2nd: 60.0%
(9/15) | |
| Body and tail | 59.5% (25/42) | 83.3% (65/78) | 0.005 |
| | ERCP 1st: 38.5%
(10/26) | |
| | EUS-FNA 1st: 96.2%
(50/52) | |
| | EUS-FNA 2nd: 71.4%
(5/7) | |
Comparison of consistency and frequency
of complications between the two groups
The incidence of complications was 4.95% (5/101) in
group A and 3.09% (5/162) in group B (P=0.448). When the patients
in group B who did not consent to EUS-FNA after ERCP were excluded,
the incidence of complications in group B was 3.73% (5/134). In
group A, the complications included PEP in 4 cases (mild in 3 and
moderate in 1) and hemorrhage due to endoscopic sphincterotomy in 1
case; in group B, PEP was observed in 4 cases (mild in 3 and
moderate in 1) and post-EUS-FNA pancreatitis (moderate) in 1 case.
Hyperamylasemia developed in 9 cases in each group. All the
complications improved with conservative treatment, without severe
cases or fatalities (Table
III).
 | Table IIIComparison of complication frequency
between groups A and B. |
Table III
Comparison of complication frequency
between groups A and B.
| Complication
frequency | Group A | Group B | P-value |
|---|
| Total | 4.95% (5/101) | 3.09% (5/162) | 0.4481 |
| Due to ERCP, n |
| Pancreatitis | 4a | 4b | |
| Hemorrhage | 1 | | |
| Due to EUS-FNA,
n |
| Pancreatitis | - | 1c | |
Discussion
The PC diagnostic accuracy rate based on diagnostic
imaging has improved as a result of advances in various diagnostic
imaging equipment over the last few years; whether histological
diagnosis is necessary when distant metastasis is present remains
debatable. However, against a background of cases in which it is
difficult to perform a differential diagnosis from inflammatory
diseases and due to the recent advances in the multidisciplinary
treatment of PC, it is crucial to obtain a histopathological
diagnosis prior to treatment (5).
Obtaining a pathological basis is crucial for undertaking
chemotherapy and selecting the appropriate medication, particularly
in cases with unresectable disease, in which a definitive
pathological diagnosis is impossible.
Percutaneous and open biopsy have long been used to
obtain PC pathology specimens; however, endoscopic non-invasive
approaches have recently become mainstream. ERCP-guided specimen
collection and EUS-FNA are currently preferred as endoscopic
collection methods, although the ERCP-guided method has been used
longer. ERCP-guided direct collection of specimens from the bile
and pancreatic duct is considered a viable diagnostic method from
the standpoint of ordinary PCs arising from the pancreatic duct
epithelium; however, there is a wide variation (33–92%) in the
sensitivity of ERCP-guided specimen collection among institutions
and its usefulness is not consistent (6–9).
Consequently, various modifications have been
attempted to improve diagnostic performance. Pancreatic juice
cytology, in which secretin was used to forcibly stimulate
pancreatic juice secretion, was previously performed, with reported
good results, i.e., 30–79% (10).
However, since 2004, when secretin became unavailable, reports on
brush cytology, in which specimens are collected by forcibly
brushing the pancreatic duct, have become more common. The
diagnostic performance of brush cytology was reported to be 30–93%,
although there have been occasional reports of even more ingenious
modifications (7). We previously
reported that, when we collected pancreatic juice that had
accumulated after brushing, the sensitivity improved from 62 to
73.7%; the diagnostic performance was also improved (9). We hypothesized that these results
were attributable to mechanically stripping the pancreatic duct
epithelium, which enabled the collection of numerous fresh cells,
thereby minimizing degeneration by pancreatic enzymes (11). Uehara et al (6) reported achieving 92% sensitivity by a
method in which scraping was performed with a guidewire.
Furthermore, Kimura et al (8) reported that it was possible to
perform cytology several times by following ERCP with placement of
an endoscopic naso-pancreatic drainage tube, with a sensitivity of
62%.
After a report of EUS-FNA for PC by Vilmann et
al (3) in 1992, EUS-FNA became
widely adopted, primarily in Western countries. The results for
diagnostic performance regarding pancreatic mass lesions revealed a
sensitivity of 85–94%, a specificity of 100% and an accuracy of
95%, which were better compared to those with ERCP-guided cytology
(12–14).
In the present study, the sensitivity of the
ERCP-guided collection method was 60.4% in group A, 45.7% in group
B, and 53.3% overall; thus, we obtained results consistent with
those in previous reports. By contrast, the sensitivity of EUS-FNA
in group B was 87.0% (EUS-FNA 1st, 94.3% and EUS-FNA 2nd, 63.6%)
and its diagnostic performance was superior to that of ERCP-guided
cytology.
The fact that the cases in which ERCP-guided
cytology was performed in group B were restricted to pancreatic
head lesions that required EBS appears to have been a factor
affecting the differences in the diagnostic performance of the
ERCP-guided collection method between groups A and B. This
difference may be due to the fact that the main pancreatic duct was
obstructed in a number of the pancreatic head cancers and it was
impossible to collect an adequate specimen by brushing.
The presence of more pancreatic head lesions in the
EUS-FNA 2nd cases in group B may have been another factor
responsible for the difference in sensitivity between EUS-FNA 1st
and EUS-FNA 2nd cases in group B. A transduodenal approach is often
used to perform EUS-FNA for pancreatic head lesions and puncture
manoeuvres may prove difficult, as the tip of the scope bends
sharply as a result of the endoscope angle. Therefore, diagnostic
performance appears to be reduced by a technical problem (15,16).
Haba et al (17) also
reported poorer diagnostic accuracy for pancreatic head lesions
compared to body and tail lesions. There was also a report
according to which a 25G needle that makes puncture manoeuvres
easier compared to conventional needles was recently developed and
the specimen collection rate and diagnostic performance for
pancreatic head lesions have been improved (18); however, additional testing is
required. ROSE was also reported to improve diagnostic accuracy
(19), contribute to increasing
diagnostic performance, reducing the number of punctures and
reducing the complication rate (20).
The incidence of complications attributable to ERCP
is considered to be high for an endoscopic procedure and the
incidence of PEP, in particular, was reported to be 2–9% (21–23).
By contrast, the incidence of complications in patients who undergo
EUS-FNA was reported to be <2%, making EUS-FNA a relatively safe
procedure (24,25). In the present study, the overall
incidence of PEP was 4%, but the complications of EUS-FNA included
only one case of pancreatitis (incidence rate of 1%). Tumor seeding
as a result of the puncture is a rare complication of EUS-FNA; to
date, a total of 4 cases have been reported, i.e., a case of
seeding of the abdominal cavity by a mucinous cystic tumor of the
pancreas (4), a case of pancreatic
metastasis by a malignant melanoma (26), seeding of the puncture tract by a
pancreatic tail cancer (27) and
seeding of a metastatic mediastinal lymph node after a
transesophageal puncture (28).
However, Ikezawa et al (29) reported that EUS-FNA of the pancreas
does not increase the risk of seeding. It may be possible to reduce
the problem of cancer seeding by EUS-FNA by excluding special
cases, such as mucinous cystic tumors of the pancreas. In addition,
a study conducted by Matsumoto et al (30) on a group of PC patients who were
treated by chemotherapy, reported that there was no difference in
the incidence rates of peritonitis carcinomatosa or ascites between
patients who had undergone EUS-FNA and those who had not.
Therefore, we do not consider EUS-FNA as disadvantageous, at least
for patients with unresectable PC who are candidates for
chemotherapy.
Certain recent studies reported that ERCP and
EUS-FNA performed on the same day may be more efficient and
cost-effective (31,32). Those reports taken together with
the results of the present study suggest that it may be more
efficient to perform EUS-FNA on the same day as EBS in unresectable
PC cases that are complicated by obstructive jaundice.
In conclusion, there was a significant difference in
the endoscopic cytological diagnosis cancer-positive rate in
patients with unresectable PC prior to and after the introduction
of EUS-FNA, with the results improving after the introduction of
EUS-FNA. In addition, although the difference in the complication
rate prior to and after the introduction of EUS-FNA was not
significant, a number of the complications were attributable to the
ERCP procedure.
References
|
1
|
Lee JG and Leung J: Tissue sampling at
ERCP in suspected pancreatic cancer. Gastrointest Endosc Clin N Am.
8:221–235. 1998.PubMed/NCBI
|
|
2
|
Howell DA, Parsons WG, Jones MA, Bosco JJ
and Hanson BL: Complete tissue sampling of biliary strictures at
ERCP using a new device. Gastrointest Endosc. 43:498–502. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vilmann P, Jacobsen GK, Henriksen FW and
Hancke S: Endoscopic ultrasonography with guided fine needle
aspiration biopsy in pancreatic disease. Gastrointest Endosc.
38:172–173. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirooka Y, Goto H, ltoh A, et al: Case of
intraductal papillary mucinous tumor in which endosonography-guided
fine-needle aspiration biopsy caused dissemination. J Gastroenterol
Hepatol. 18:1323–1324. 2003. View Article : Google Scholar
|
|
5
|
Kichise J, Suzuki E, Hirokawa S, Kitamura
H and Nakashima F: Significance of pathological examination in
biliary tract and pancreatic cancer. J Biliary Tract Pancreas.
31:809–813. 2010.(In Japanese).
|
|
6
|
Uehara H, Tatsunami K, Masuda E, et al:
Scraping cytology with a guidewire for pancreatic-ductal
strictures. Gastrointest Endosc. 70:52–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arizumi T, Tada M, Togawa O, et al:
Efficacy of combinatorial diagnosis of pancreatic cancer;
Combination of variety of diagnostic modalities and specimen
collecting method. Gastroenterology. 128(Suppl 2): A4702005.
|
|
8
|
Kimura K, Furukawa Y, Yamasaki S, et al: A
study of the usefulness of pancreatic juice cytology obtained via
an endoscopic nasal pancreatic drainage (ENPD) tube. Jpn J
Gastroenterol. 108:928–936. 2011.(In Japanese).
|
|
9
|
Okabe Y, Naito Y, Kawahara A, et al: The
Management of endoscopic transpapillary cytology for the pancreatic
cancer. J Biliary Tract Pancreas. 27:157–161. 2006.(In
Japanese).
|
|
10
|
Nakaizumi A, Tatsuta M, Uehara H, et al:
Cytologic examination of pure pancreatic juice in the diagnosis of
pancreatic carcinoma. The endoscopic retrograde intraductal
catheter aspiration cytologic technique. Cancer. 70:2610–2614.
1992. View Article : Google Scholar
|
|
11
|
Naito Y, Okabe Y, Kawahara A, Taira T,
Kusano H and Kage M: Study on the cytology of the pancreatic duct
by different sampling. Jpn Soc Clin Cytol. 46:7–11. 2007.(In
Japanese).
|
|
12
|
Yamao K, Sawaki A, Mizuno N, Shimizu Y,
Yatabe Y and Koshikawa T: Endoscopic ultrasound-guided fine-needle
aspiration biopsy(EUS-FNAB): past, present, and future. J
Gastroenterol. 40:1013–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fisher L, Segarajasingam DS, Stewart C,
Deboer WB and Yusoff F: Endoscopic ultrasound guided fine needle
aspiration of solid pancreatic lesions: Performance and outcomes. J
Gastroenterol Hepatol. 24:90–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hewitt MJ, McPhail MJ, Possamai L, Dhar A,
Vlavianos P and Monahan KJ: EUS-guided FNA for diagnosis of solid
pancreatic neoplasma: a meta-analysis. Gastrointest Endosc.
75:319–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Larghi A, Verna EC, Stavropoulos SN,
Rotterdam H, Lightdale CJ and Stevens PD: EUS-guided trucut needle
biopsies in patients with solid pancreatic masses: a prospective
study. Gastrointest Endosc. 59:185–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Itoi T, Tsuchiya T, Itokawa F, Sofuni A,
Kurihara T, Tsuji S and Ikeuchi N: Histological diagnosis by
EUS-guided fine-needle aspiration biopsy in pancreatic solid masses
without on-site cytopathologist: a single-center experience. Dig
Endosc. 23(Suppl 1): 34–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haba S, Yamao K, Bhatia V, et al:
Diagnostic ability and factors affecting accuracy of endoscopic
ultrasound-guided fine needle aspiration for pancreatic solid
lesions: Japanese large single center experience. J Gastroenterol.
48:973–981. 2013. View Article : Google Scholar
|
|
18
|
Sakamoto H, Kitano M, Komaki T, et al:
Prospective comparative study of the EUS guided 25-gauge FNA needle
with the 19-gauge Trucut needle and 22-gauge FNA needle in patients
with solid pancreatic masses. J Gastroenterol Hepatol. 24:384–390.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hikichi T, lrisawa A, Bhutani MS, et al:
Endoscopic ultrasound-guided fine-needle aspiration of solid
pancreatic masses with rapid on-site cytological evaluation by
endosonographers without attendance of cytopathologists. J
Gastroenterol. 44:322–328. 2009. View Article : Google Scholar
|
|
20
|
Iglesias-Garcia J, Dominguez-Munoz JE,
Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A and
Forteza-Vila J: Influence of on-site cytopathology evaluation on
the diagnostic accuracy of endoscopic ultrasound-guided dine needle
aspiration (EUS-FNA) of solid pancreatic masses. Am J
Gastroenterol. 106:1705–1710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Freeman ML, DiSario JA, Nelson DB, et al:
Risk factors for post-ERCP pancreatitis: a prospective, multicenter
study. Gastrointest Endosc. 54:425–434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Li ZS, Liu F, et al: Risk factors
for ERCP related complications: a prospective multicenter study. Am
J Gastroenterol. 104:31–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williams EJ, Taylor S, Fairclough P, et
al: Risk factors for complication following ERCP; results of a
large-scale, prospective multicenter study. Endoscopy. 39:793–801.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamao K: Complications of endoscopic
ultrasound guided fine-needle aspiration biopsy (EUS-FNAB) for
pancreatic lesions. J Gastroenterol. 40:921–923. 2005. View Article : Google Scholar
|
|
25
|
Polkowski M, Larghi A, Weynand B,
Boustlère C, Glovannini B, Pujol B and Dumonceau JM; European
Society of Gastrointestinal Endoscopy (ESGE). Learning, techniques,
and complications of endoscopic ultrasound (EUS)-guided sampling in
gastroenterology: European Society of Gastrointestinal Endoscopy
(ESGE) Technical Guideline. Endoscopy. 44:190–206. 2012. View Article : Google Scholar
|
|
26
|
Shah JN, Fraker D, Guerry D, Feldman M and
Kochman ML: Melanoma seeding of an EUS-guided fine needle track.
Gastrointest Endosc. 59:923–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paquin SC, Gariépy G, Lepanto L, et al: A
first report tumor seeding because of EUS-guided FNA of a
pancreatic adenocarcinoma. Gastrointest Endosc. 61:610–611. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doi S, Yasuda I, lwashita T, et al: Needle
tract implantation on the esophageal wall after EUS-guided FNA of
metastatic mediastinal lymphadenopathy. Gastrointest Endosc.
67:988–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikezawa K, Uehara H, Sakai A, et al: Risk
of peritoneal carcinomatosis by endoscopic ultrasound-guided fine
needle aspiration for pancreatic cancer. J Gastroenterol.
48:966–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsumoto K, Yamao K, Ohashi K, et al: The
clinical utility of EUS-guided fine-needle aspiration (EUS-FNA) for
pancreatic lesions. J Jpn Pancreas Soc. 17:485–491. 2002.(In
Japanese).
|
|
31
|
Aslanian HR, Estrada JD, Rossi F, Dziura
J, Jamidar PA and Siddiqui UD: Endoscopic ultrasound and endoscopic
retrograde cholangiopancreatography for obstructing pancreas head
masses: combined or separate procedures? J Clin Gastroenterol.
45:711–713. 2011. View Article : Google Scholar
|
|
32
|
Ross WA, Wasan SM, Evans DB, et al:
Combined EUS with FNA and ERCP for the evaluation of patients with
obstructive jaundice from presumed pancreatic malignancy.
Gastrointest Endosc. 68:461–466. 2008. View Article : Google Scholar : PubMed/NCBI
|