|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang X, Ye X, Dong L, et al: Human
epididymis protein 4 (HE4) as a serum tumor biomarker in patients
with ovarian carcinoma. Int J Gynecol Cancer. 21:852–858. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fountain J, Trimble E and Birrer MJ:
Summary and discussion of session recommendations. Gynecol Oncol.
103:S23–S25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buamah P: Benign conditions associated
with raised serum CA-125 concentration. J Surg Oncol. 75:264–265.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yurkovetsky Z, Skates S, Lomakin A, et al:
Development of a multimarker assay for early detection of ovarian
cancer. J Clin Oncol. 28:2159–2166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cree IA: Improved blood tests for cancer
screening: general or specific? BMC Cancer. 11:4992011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drapkin R, von Horsten HH, Lin Y, et al:
Human epididymis protein 4 (HE4) is a secreted glycoprotein that is
overexpressed by serous and endometrioid ovarian carcinomas. Cancer
Res. 65:2162–2169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galgano MT, Hampton GM and Frierson HF Jr:
Comprehensive analysis of HE4 expression in normal and malignant
human tissues. Mod Pathol. 19:847–853. 2006.PubMed/NCBI
|
|
9
|
Partheen K, Kristjansdottir B and
Sundfeldt K: Evaluation of ovarian cancer biomarkers HE4 and CA-125
in women presenting with a suspicious cystic ovarian mass. J
Gynecol Oncol. 22:244–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li F, Tie R, Chang K, et al: Does risk for
ovarian malignancy algorithm excel human epididymis protein 4 and
CA125 in predicting epithelial ovarian cancer: a meta-analysis. BMC
Cancer. 12:2582012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: the PRISMA statement. J Clin Epidemiol.
62:1006–10012. 2009. View Article : Google Scholar
|
|
12
|
Stroup DF, Berlin JA, Morton SC, et al:
Meta-analysis of observational studies in epidemiology: a proposal
for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar
|
|
13
|
Whiting PF, Rutjes AW, Westwood ME, et al:
QUADAS-2: a revised tool for the quality assessment of diagnostic
accuracy studies. Ann Intern Med. 155:529–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bossuyt PM, Reitsma JB, Bruns DE, et al:
Standards for Reporting of Diagnostic Accuracy: Towards complete
and accurate reporting of studies of diagnostic accuracy: the STARD
initiative. Standards for Reporting of Diagnostic Accuracy. Clin
Chem. 49:1–6. 2003. View
Article : Google Scholar
|
|
15
|
Devillé WL, Buntinx F, Bouter LM, et al:
Conducting systematic reviews of diagnostic studies: didactic
guidelines. BMC Med Res Methodol. 2:92002.PubMed/NCBI
|
|
16
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pepe MS, Feng Z, Janes H, et al: Pivotal
evaluation of the accuracy of a biomarker used for classification
or prediction: standards for study design. J Natl Cancer Inst.
100:1432–1438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glas AS, Lijmer JG, Prins MH, et al: The
diagnostic odds ratio: a single indicator of test performance. J
Clin Epidemiol. 56:1129–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walter SD: Properties of the summary
receiver operating characteristic (SROC) curve for diagnostic test
data. Stat Med. 21:1237–1256. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huedo-Medina TB, Sánchez-Meca J,
Marín-Martínez F and Botella J: Assessing heterogeneity in
meta-analysis: Q statistic or I2index? Psychol Methods.
11:193–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dinnes J, Deeks J, Kirby J and Roderick P:
A methodological review of how heterogeneity has been examined in
systematic reviews of diagnostic test accuracy. Health Technol
Assess. 9:1–113. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
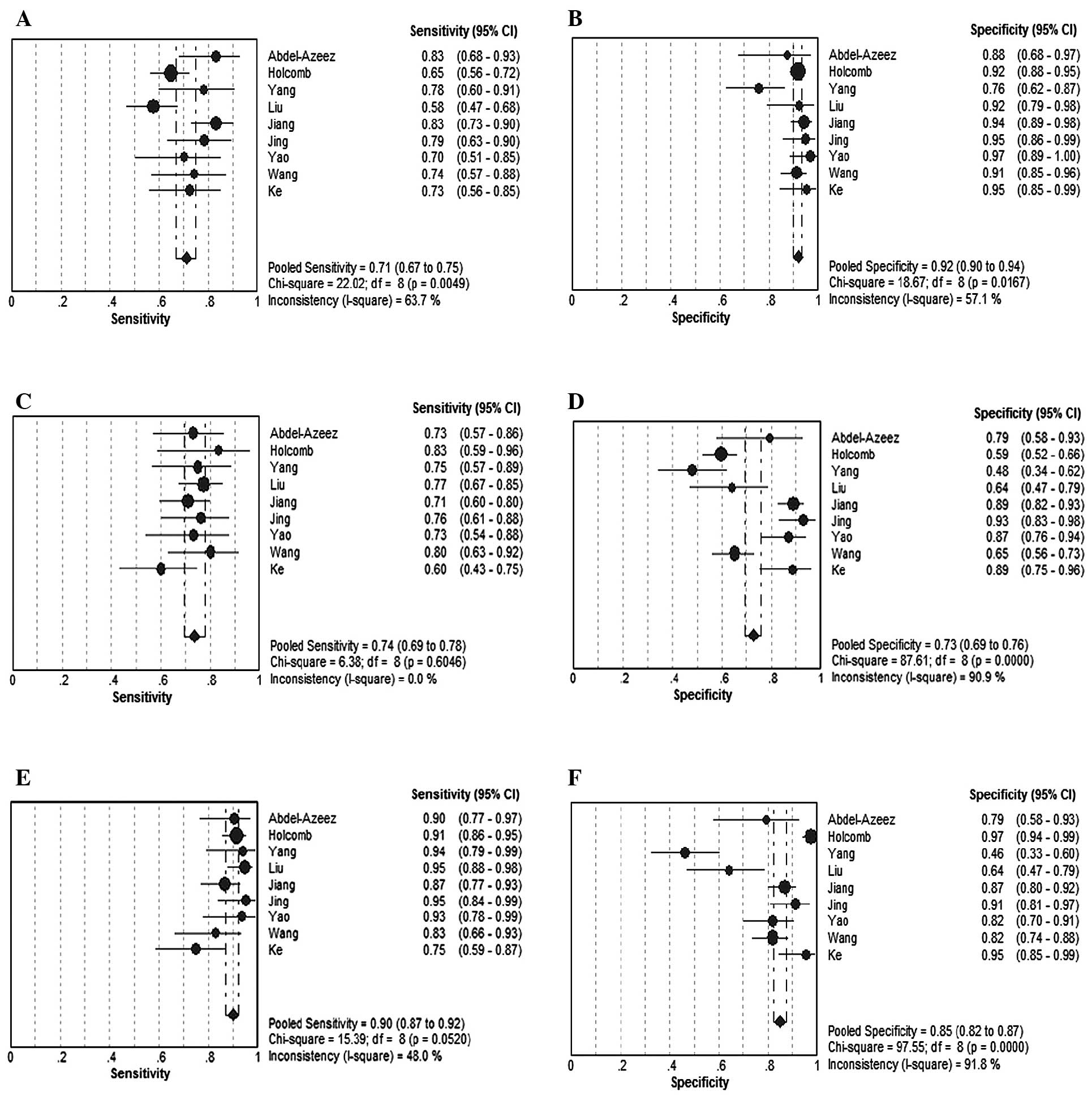
Abdel-Azeez HA, Labib HA, Sharaf SM and
Refai AN: HE4 and mesothelin: novel biomarkers of ovarian carcinoma
in patients with pelvic masses. Asian Pac J Cancer Prev.
11:111–116. 2010.PubMed/NCBI
|
|
23
|
Anastasi E, Marchei GG, Viggiani V, et al:
HE4: a new potential early biomarker for the recurrence of ovarian
cancer. Tumor Biol. 31:113–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersen MR, Goff BA, Lowe KA, et al: Use
of a Symptom Index, CA125, and HE4 to predict ovarian cancer.
Gynecol Oncol. 116:378–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Gorp T, Cadron I, Despierre E, et al:
HE4 and CA125 as a diagnostic test in ovarian cancer: prospective
validation of the Risk of Ovarian Malignancy Algorithm. Br J
Cancer. 104:863–870. 2011.PubMed/NCBI
|
|
26
|
Holcomb K, Vucetic Z, Miller MC and Knapp
RC: Human epididymis protein 4 offers superior specificity in the
differentiation of benign and malignant adnexal masses in
premenopausal women. Am J Obstet Gynecol. 205:358.e1–358.e6. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jacob F, Meier M, Caduff R, et al: No
benefit from combining HE4 and CA125 as ovarian tumor markers in a
clinical setting. Gynecol Oncol. 121:487–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Montagnana M, Danese E, Ruzzenente O, et
al: The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating
the risk of epithelial ovarian cancer in women presenting with
pelvic mass: is it really useful? Clin Chem Lab Med. 49:521–525.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moore RG, Brown AK, Miller MC, et al: The
use of multiple novel tumor biomarkers for the detection of ovarian
carcinoma in patients with a pelvic mass. Gynecol Oncol.
108:402–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nolen B, Velikokhatnaya L, Marrangoni A,
et al: Serum biomarker panels for the discrimination of benign from
malignant cases in patients with an adnexal mass. Gynecol Oncol.
117:440–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park Y, Kim Y, Lee EY, et al: Reference
ranges for HE4 and CA125 in a large Asian population by automated
assays and diagnostic performances for ovarian cancer. Int J
Cancer. 130:1136–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong L, Cheng XH, Ye X, et al: The values
of serum human epididymis secretory protein 4 and CA(125) assay in
the diagnosis of ovarian malignancy. Chin J Obstet Gynecol.
43:931–936. 2008.(In Chinese).
|
|
33
|
Wu XW, Fu GY, Wang R and Shi XQ:
Significance of using combined assays of serum human epididymis
secretory protein 4, CA125 and ROMA in the diagnosis of ovarian
malignancy and pelvic mass. J Basic Clin Oncol. 5:252012.
|
|
34
|
Yang C, Song ML, Zhong HB, et al: The
differential diagnostic value of HE4, CA125 and the risk of ovarian
malignancy aligorithm in ovarian tumor. Suzhou Univ J Med Sci.
30:42010.
|
|
35
|
Huang S and Zeng Q: The clinical value of
HE4 and CA125 combined with sB7-H4 in early diagnosis of ovarian
cancer. Hebei Med. 17:4432011.(In Chinese).
|
|
36
|
Li Q, Song X, Wu Q, et al: Clinical
application of combined HE4 and CA125 differentiate malignant
ovarian tumors from ovarian endometriotic cysts. Mod Oncol.
21:22013.
|
|
37
|
Liu G, Wang A, Liu Q, et al: Combined
detection of serum CA125 and human epididymis protein 4 levels in
ovarian cancer. Chin J Clin Lab Sci. 28:119–121. 2010.(In
Chinese).
|
|
38
|
Jiang DL, Sun JM, Cai LL, et al: Changes
and clinical significance of serum HE4 in patients with ovarian
cancer. J Pract Med. 26:142010.
|
|
39
|
Jing XG, Wang GJ, Pei YX, et al:
Diagnostic value of combined measurement of CA125, HE4 and imaging
examination patients with epithelial ovarian cancer. J Third Mil
Med Univ. 33:62011.
|
|
40
|
Yao YX and Hong W: Clinical significance
of detecting serum HE4, CA125 and CA724 levels in diagnoses of
ovarian malignancies. Labeled Immunoassays Clin Med. 19:32012.(In
Chinese).
|
|
41
|
Yao YL, Liu Q and Li XY: The diagnostic
values of combined determination of serum tumor markers HE4, TPS
and CA125 levels in patients with ovarian cancer. J Radioimmunol.
23:42010.(In Chinese).
|
|
42
|
Wang KY, Leng JH, Zheng H and Jiang LH:
Studies on value of combination of human epididymis protein 4 and
CA125 in patients with ovarian cancer. Chin J Health Lab Technol.
20:1139–1140. 2010.
|
|
43
|
Li ZJ, Zheng YQ and Xu XF: Clinic value of
HE4, CA125 combined with risk of ovarian malignancy algorithm
(ROMA) in the diagnosis for ovarian cancer. J Chin Oncol.
19:219–222. 2013.
|
|
44
|
Ke and Liu F: Serum HE4 and CA125 in the
diagnosis of ovarian cancer. Mod Hosp. 10:52010.
|
|
45
|
Lin YY, Chen Y, Hu MH, et al: Significance
of HE4 detection for diagnosis of ovarian cancer as compared with
CA125 in 69 cases. Curr Immun. 33:12013.
|
|
46
|
Moore RG and Bast RC Jr: How do you
distinguish a malignant pelvic mass from a benign pelvic mass?
Imaging, biomarkers, or none of the above. J Clin Oncol.
25:4159–4161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin J, Qin J and Sangvatanakul V: Human
epididymis protein 4 for differential diagnosis between benign
gynecologic disease and ovarian cancer: a systematic review and
meta-analysis. Eur J Obstet Gynecol Reprod Biol. 167:81–85. 2013.
View Article : Google Scholar
|
|
48
|
Yu S, Yang HJ, Xie SQ and Bao YX:
Diagnostic value of HE4 for ovarian cancer: a meta-analysis. Clin
Chem Lab Med. 50:1439–1446. 2012.PubMed/NCBI
|