Introduction
Gastric cancer (GC) is one of the most common
cancers worldwide, accounting for ~700,000 deaths annually
(1). The incidence of GC among
elderly individuals has gradually increased due to the improvement
in living conditions, extended life span and aging of the
population. As recently reported, the majority of the new cases of
GC occurred in elderly individuals (2).
The symptoms of GC in elderly patients are generally
atypical and are often masked by concurrent diseases. Elderly
patients do not usually tolerate surgery as well and often suffer
from multiple postoperative complications, hence the poor prognosis
(3). Therefore, gastrectomy may be
a challenging surgical procedure for elderly patients with GC.
Laparoscopic radical gastrectomy (LRG) was first
introduced in 1994 and has since been considered to be a viable
surgical option for GC (4). This
procedure is associated with minimal postoperative pain, low degree
of trauma, rapid recovery of gastrointestinal function and reduced
hospital stay. In addition, this procedure has only a minimal
impact on immune function and is thus associated with better
survival and improved quality of life. Therefore, LRG has become
one of the optimal surgical approaches for the treatment of GC.
Clinical trials in younger patients indicated that
LRG is associated with similar or fewer intraoperative and
postoperative complications compared to open surgery (5–7).
However, the safety profile, feasibility and curative effects of
LRG have not been clearly determined in elderly patients. In the
present retrospective study, we compared the benefits,
complications and patient survival profile between elderly patients
who underwent a laparoscopic procedure and those who underwent open
surgery in a single centre in China.
Materials and methods
Patients
This study included 108 patients aged >70 years
who underwent radical gastrectomy at the General Hospital of
Lanzhou Military Region between June, 2008 and March, 2009. All the
patients had an American Society of Anesthesiologists score ≥IV
(8) and were considered to be
suitable for surgery. In all the cases, surgery was performed by
the same surgical team and the diagnosis was confirmed by
postoperative pathology.
No patients exhibited evidence of remote metastasis
on gastroscopy, computed tomography scan or magnetic resonance
imaging examination. No patients had received any immunotherapy or
had undergone preoperative chemo- and/or radiotherapy within 6
months of surgery. Furthermore, no patients experienced a switch
from laparoscopic surgery to an open approach intraoperatively.
Clinical manifestations
All the patients presented with various degrees of
abdominal discomfort. The major clinical manifestations included
upper quadrant abdominal pain or bloating (n=86, 79.6%), loss of
appetite (n=64, 59.3%), fatigue or weight loss (n=82, 75.9%),
melena (n=34, 34.5%), nausea and vomiting (n=34, 34.5%) and a
choking sensation during eating (n=23, 32.5%). The interval between
the onset of symptoms and hospital admission was <1 month in 37
patients (34.4%), 1–3 months in 45 patients (41.7%), 4–6 months in
18 patients (16.7%) and >6 months in 8 patients (7.4%).
The major comorbidities included cardiovascular or
respiratory diseases, diabetes mellitus, hypoproteinemia, central
nervous system disorders and electrolyte imbalance.
Surgical approach
The patients were randomly divided into a
laparoscopic operation group (n=54) and a traditional open surgery
group (n=54). All the patients underwent standard lymph node
dissection, according to the GC D2 radical procedure (9). In the laparoscopic group, 42 patients
underwent radical surgery and 12 received palliative surgery. The
type of procedures included total (n=31), proximal (n=14) and
distal gastrectomy (n=9). In the open surgery group, 44 patients
received radical surgery and 10 received palliative surgery. The
type of procedures in this group also included total (n=33),
proximal (n=11) and distal gastrectomy (n=10).
Postoperatively, all the patients received 6 cycles
of standard FOLFOX4 chemotherapy.
Statistical analysis
The statistical analysis was performed using SPSS
version 18.0 software (SPSS, Inc., Chicago, IL, USA). Continuous
data were analyzed using t-tests and are presented as means ±
standard deviation. Categorical data were analyzed using the
Chi-square test. The log-rank test was used to evaluate survival
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The study population included 66 men and 42 women,
aged 70–89 years (mean age, 77.6 years). No significant difference
between the laparoscopic and open surgical groups was observed in
terms of age, gender distribution, pathological type of cancer, TMN
status, clinical stage and preoperative comorbidities (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Parameters | LRG, n=54 (%) | Open surgery, n=54
(%) | χ2 | P-value |
|---|
| Age, years (mean ±
SD) | 78.6±6.5 | 76.5±7.2 | | 0.135 |
| Male/female | 36/18 | 30/24 | 1.40 | 0.236 |
| Pathological
type | | | 0.89 | 0.828 |
| Well differentiated
adenocarcinoma | 13 (24.1) | 17 (31.5) | | |
| Moderately
differentiated adenocarcinoma | 6 (11.1) | 5 (9.3) | | |
| Poorly
differentiated adenocarcinoma | 32 (59.3) | 30 (55.5) | | |
| Signet ring cell
carcinoma | 3 (5.5) | 2 (3.7) | | |
| Degree of
invasion | | | 0.38 | 0.83 |
| T1 | 5 (9.3) | 4 (7.4) | | |
| T2 | 23 (42.6) | 26 (48.1) | | |
| T3 | 26 (48.1) | 24 (44.5) | | |
| Lymph node
metastasis | | | 0.68 | 0.88 |
| N0 | 3 (5.5) | 5 (9.3) | | |
| N1 | 23 (42.6) | 21 (38.9) | | |
| N2 | 21 (38.9) | 20 (37.0) | | |
| N3 | 7 (13.0) | 8 (14.8) | | |
| Clinical staging | | | 0.34 | 0.56 |
| I/II | 24 (44.4) | 21 (38.9) | | |
| III/IV | 30 (55.6) | 33 (61.1) | | |
| Preoperative
comorbidities/conditions | | | 1.21 | 0.94 |
| Cardiovascular
disorders | 33 (61.1) | 34 (63.0) | | |
| Diabetes
mellitus | 12 (22.2) | 16 (29.6) | | |
| Respiratory
diseases | 23 (42.6) | 19 (35.2) | | |
| Hypoproteinemia | 13 (24.1) | 13 (24.1) | | |
| Cerebral
infarction | 6 (11.1) | 5 (9.3) | | |
| Electrolyte
disorders | 11 (20.4) | 13 (24.1) | | |
| Total | 46 (85.2) | 44 (81.5) | 0.27 | 0.61 |
Intraoperative and postoperative
outcomes
Except for the operative time required and the
number of lymph nodes dissected, which did not differ significantly
between the two types of surgical procedures (P-values of 0.201 and
0.167, respectively), all other major intra- and postoperative
indices were significantly superior with LRG compared to those with
open surgery. For example, compared to patients who underwent open
surgery, patients who received laparoscopic surgery exhibited a
significantly reduced intraoperative blood loss (103.0±34.4 vs.
140.6±44.4 ml, P<0.000), a significantly smaller incision size
(5.18±0.7 vs. 17.8±1.0 cm, P<0.000) and a significantly shorter
bedbound time (1.0±0.3 vs. 3.2±0.5 days, P=0.000). Further details
are shown in Table II.
 | Table IIIntraoperative and postoperative
data. |
Table II
Intraoperative and postoperative
data.
| Parameters | LRG, n=54 (mean ±
SD) | Open surgery,
n=54 | t-value | P-value |
|---|
| Intraoperative |
| Operation time
(min) | 179.4±22.5 | 173.0±28.8 | 1.288 | 0.201 |
| Blood loss (ml) | 103.0±34.4 | 140.6±44.4 | −4.917 | 0.000 |
| Lymph nodes resected
(no.) | 27.8±3.9 | 26.7±4.6 | 1.391 | 0.167 |
| Incision length
(cm) | 5.18±0.7 | 17.8±1.0 | −74.258 | 0.000 |
| Postoperative |
| Bedbound time
(days) | 1.0±0.3 | 3.2±0.5 | −29.766 | 0.000 |
| Intubation time
(days) | 0.8±0.2 | 1.6±0.7 | −7.921 | 0.000 |
| Fever (days) | 2.2±0.8 | 3.2±0.8 | −8.593 | 0.000 |
| Time to normal diet
(days) | 3.0±0.4 | 3.8±0.8 | −8.942 | 0.000 |
| Time to bowel open
(days) | 3.2±0.8 | 3.6±0.7 | −2.473 | 0.015 |
| Hospital stay
(days) | 7.0±1.3 | 9.4±1.5 | −8.987 | 0.000 |
Postoperative complications
Postoperative complications were reported in 8
patients (14.8%) who underwent laparoscopic surgery and in 16
(29.6%) who received open surgery (P=0.04). One patient who
underwent open surgery succumbed to a lung and abdominal infection
and subsequent multiple organ failure. There were no reported
deaths in the laparoscopic surgery group. Further details on
postoperative complications are listed in Table III.
 | Table IIIPostoperative complications. |
Table III
Postoperative complications.
| Complicationsa | LRG, n=54 (%) | Open surgery, n=54
(%) | P-value |
|---|
| Gastrointestinal
hypomotility | 2 (3.7) | 3 (5.6) | |
| Anastomotic
bleeding | 0 | 0 | |
| Anastomotic
leakage | 3 (5.6) | 5 (9.3) | |
| Abdominal
infection | 3 (5.6) | 6 (11.1) | |
| Poor healing of
incision | 2 (3.7) | 9 (16.7) | |
| Urinary tract
infection | 1 (1.9) | 5 (9.3) | |
| Pulmonary
infection | 3 (5.6) | 5 (9.3) | |
| Respiratory
failure | 0 | 1 (1.9) | |
| Liver/kidney
dysfunction | 2 (3.7) | 1 (1.9) | |
| Heart failure | 1 (1.9) | 1 (1.9) | |
| Deep venous
thrombosis | 0 | 0 | |
| Intestinal
obstruction | 1 (1.9) | 4 (7.4) | |
| Death | 0 | 1 (1.9) | |
| Total | 8 (14.8) | 16 (29.6) | 0.040 |
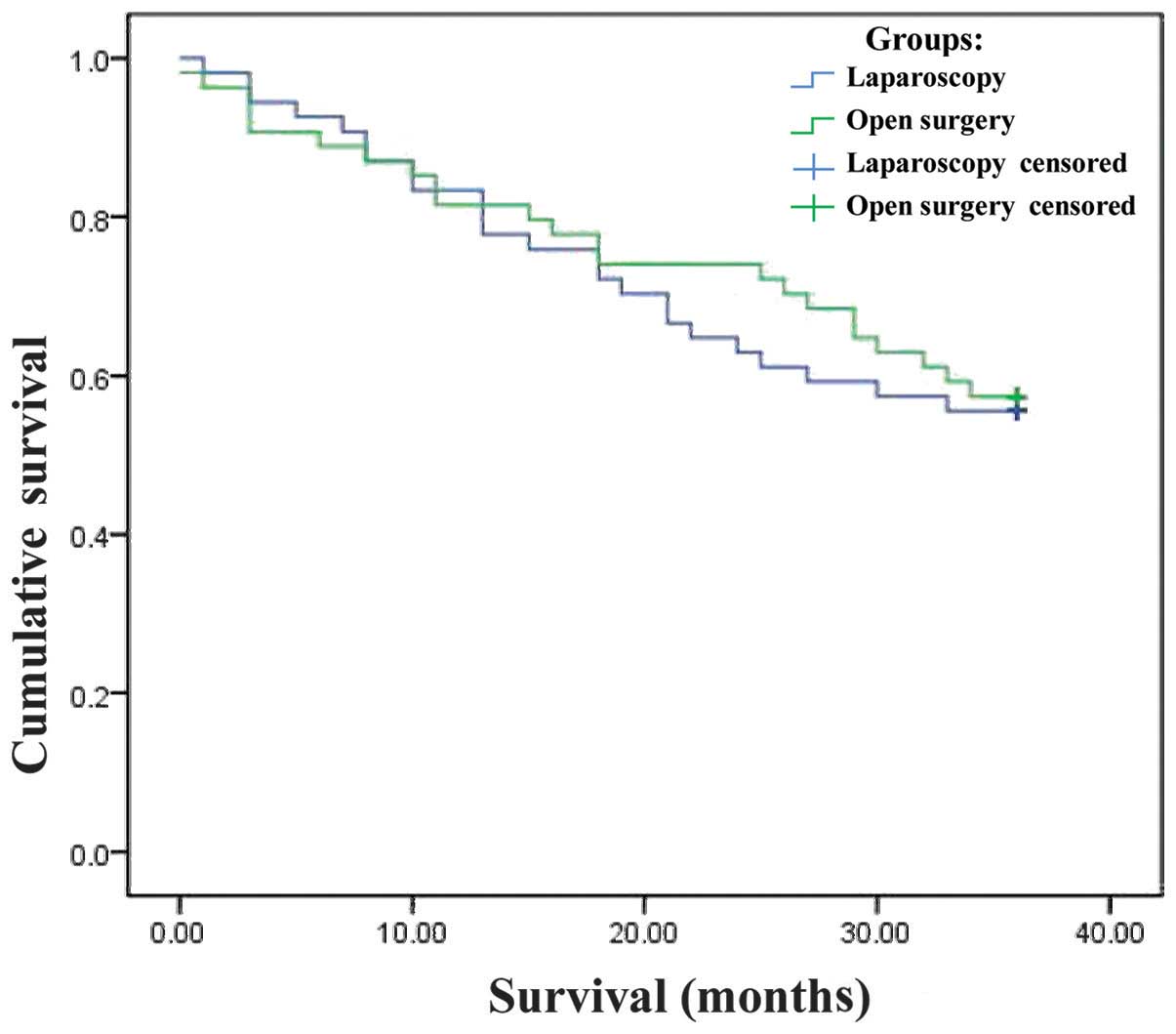
Follow-up
The patients were followed up for 3 years. The
6-month, 1- and 3-year survival rates were 92.6, 85.2 and 55.6%,
respectively, among patients who underwent laparoscopic surgery and
88.9, 81.5 and 57.4% respectively, among those who underwent open
surgery (Table IV). The median
survival was 26.8 months (95% confidence interval: 23.6–30.0) in
both groups.
 | Table IVNumber of patients who survived
during the follow-up period. |
Table IV
Number of patients who survived
during the follow-up period.
| Time (years) | LRG, n=54 (%) | Open surgery, n=54
(%) | χ2 | P-value |
|---|
| 1/2 | 50 (92.6) | 48 (88.9) | 0.441 | 0.507 |
| 1 | 46 (85.2) | 44 (81.5) | 0.267 | 0.606 |
| 3 | 30 (55.6) | 31 (57.4) | 0.038 | 0.846 |
The Kaplan-Meier survival curves for the two groups
are shown in Fig. 1. By the
log-rank test, there was no significant difference in survival
between the two surgical techniques (χ2=0.079;
P=0.779).
All the patients who underwent palliative surgery
succumbed to disease progression during the 3-year follow-up
period. The causes of death in the laparoscopic surgery group
included cardiovascular accidents (n=10), extensive abdominal and
peritoneal metastasis (n=8) and liver metastasis (n=3). Of the
patients who underwent palliative treatment through open surgery, 7
died due to cardiovascular accidents, 9 due to extensive abdominal
and peritoneal metastasis and 2 due to liver metastasis (data not
shown).
Discussion
The symptoms of GC are usually not overt in elderly
patients, partly due to an underlying reduction in physiological
functionality and partly because the symptoms are often attributed
to other comorbidities. Elderly patients with early-stage GC are
often either asymptomatic or present with non-specific symptoms,
such as dull pain in the upper quadrant, abdominal discomfort, loss
of appetite and weight loss (10).
Therefore, at the time of diagnosis, the disease has often reached
an advanced stage and is difficult to treat. Consequently, in all
suspected GC cases, endoscopy and upper gastrointestinal barium
meal examination are required for early diagnosis.
Surgery remains the major therapeutic approach for
GC. However, elderly patients may not be able to tolerate surgery
and are more susceptible to postoperative complications and
increased mortality risk as a result of multiple concurrent
diseases and declining organ function.
A number of elderly patients exhibit a poor
compensatory ability due to a weakened immune system, which may be
exacerbated by long-term nutritional insufficiency and digestive
tract hemorrhage. The presence of multiple coexisting diseases
highlights the significance of a thorough preoperative
investigation of heart, lung, liver and kidney function, as well as
determination of the coagulation profile and blood glucose levels.
Malnutrition and anemia should be corrected prior to surgery and
steps should be taken to improve the respiratory function, such as
quitting smoking and physiotherapy to improve effective coughing
and expectoration. Palliative surgery should be considered for
patients with multiple, severe concurrent diseases.
The first laparoscopic partial gastrectomy for
early-stage GC was undertaken in 1994 in an attempt to reduce the
iatrogenic trauma associated with gastrectomy (11). This was followed 5 years later by
LRG for advanced GC, including D2 lymph node dissection (12). A retrospective study of 1,294
patients undergoing gastrectomy at 16 medical centers in Japan
reported that laparoscopic and open surgery were of equivalent
therapeutic value, feasibility and safety (13). Based on those findings,
laparoscopic surgery has been accepted as a novel minimally
invasive surgical option for GC patients. However, elderly patients
are generally less tolerant to the pneumoperitoneum that forms an
essential part of the laparoscopic procedure. The relatively long
operative time required for LRG has also limited its application in
elderly patients.
Our previous experience with laparoscopic surgery
suggests that this type of procedure is able to preserve the
integrity of the dissected lymph nodes and lymph ducts, provided
that any associated lymphatic and connective tissues are removed en
bloc. Laparoscopy facilitates this part of the operative procedure,
as the surgical view is amplified, enabling complete removal of all
malignant tissue and reducing the risk of recurrence.
Our present study revealed that laparoscopic surgery
is advantageous over open surgery in a number of important
postoperative endpoints (Table
III), which is is partially in line with previously reported
data (14). Compared to open
surgery, laparoscopic surgery was found to be associated with less
intraoperative blood loss, rapid intestinal recovery (i.e., shorter
time to bowel function following surgery), less bedbound time, less
intubation time and less overall hospital stay. Unlike previous
reports suggesting that laparoscopy requires a significantly longer
time compared to open gastrectomy in elderly patients, our data
demonstrated that the mean operative time was longer by only 6.4
minutes with laparoscopic surgery compared to that with open
surgery; this difference was marginal and of no statistic
significance. As the laparoscopic procedure is more complicated
compared to open surgery, a longer learning curve is required.
However, to certain extent, the duration of surgery depends on the
experience of the surgeon and the complexity of each case.
In line with previous studies, our analysis
indicated that laparoscopic surgery is associated with fewer
postoperative complications compared to open surgery. A recent
study in GC patients reported a postoperative complication rate of
25.3% with laparoscopic surgery (n=629) and 40.1% with open surgery
(n=1,002). In our study, the overall postoperative complication
rate was 14.8% with laparoscopic surgery (n=8) and 29.6% with open
surgery (n=16). When used for radical and palliative gastrectomy
with lymph node dissection, the two procedures had a similar impact
on long-term survival, which is consistent with previous studies
(15,16).
In summary, LRG appears to be superior to open
surgery in improving patient survival and quality of life when
combined with appropriate perioperative care in elderly patients
with GC.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Bai Y and Li ZS: Endoscopic,
clinicopathological features and prognosis of very young patients
with gastric cancer. J Gastroenterol Hepatol. 26:1626–1629. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saif MW, Makrilia N, Zalonis A, et al:
Gastric cancer in the elderly: an overview. Eur J Surg Oncol.
36:709–717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitano S, Iso Y, Moriyama M, et al:
Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc.
4:146–148. 1994.
|
|
5
|
Pugliese R, Maggioni D, Sansonna F, et al:
Outcomes and survival after laparoscopic gastrectomy for
adenocarcinoma. Analysis on 65 patients operated on by conventional
or robot-assisted minimal access procedures. Eur J Surg Oncol.
35:281–288. 2009. View Article : Google Scholar
|
|
6
|
Hwang SI, Kim HO, Yoo CH, et al:
Laparoscopic-assisted distal gastrectomy versus open distal
gastrectomy for advanced gastric cancer. Surg Endosc. 23:1252–1258.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Francescutti V, Choy I, Biertho L, et al:
Gastrectomy and esophagogastrectomy for proximal and distal gastric
lesions: a comparison of open and laparoscopic procedures. Surg
Innov. 16:134–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Munish M, Sharma V, Yarussi KM, et al: The
use of practice guidelines by the American Society of
Anesthesiologists for the identification of surgical patients at
high risk of sleep apnea. Chron Respir Dis. 9:221–230. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uyama I, Sakurai Y, Komori Y, et al: The
advances of laparoscopic treatment for gastric cancer. Cancer &
Chemotherapy. 34:21–24. 2007.(In Japanese).
|
|
10
|
Ramesh HS, Pope D, Gennari R, et al:
Optimising surgical management of elderly cancer patients. World J
Surg Oncol. 3:172005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohgami M, Otani Y, Kumai K, et al:
Laparoscopic wedge resection of the stomach for early gastric
cancer using a lesion-lifting-method: curative and minimally
invasive treatment. Zentralbl Chir. 123:465–468. 1998.(In
German).
|
|
12
|
Uyama I, Sugioka A, Fujita J, et al:
Purely laparoscopic pylorus-preserving gastrectomy with
extraperigastric lymphadenectomy for early gastric cancer: a case
and technical report. Surg Laparosc Endosc Percutan Tech.
9:418–422. 1999. View Article : Google Scholar
|
|
13
|
Kitano S, Shiraishi N, Uyama I, et al: A
multicenter study on oncologic outcome of laparoscopic gastrectomy
for early cancer in Japan. Ann Surg. 245:68–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kunisaki C, Makino H, Takagawa R, et al:
Efficacy of laparoscopy-assisted distal gastrectomy for gastric
cancer in the elderly. Surg Endosc. 23:377–383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ibanez Aguirre FJ, Azagra JS, Erro
Azcarate ML, et al: Laparoscopic gastrectomy for gastric
adenocarcinoma. Long-term results. Rev Esp Enferm Dig. 98:491–500.
2006.(In English, Spanish).
|
|
16
|
Azagra JS, Ibanez-Aguirre JF, Goergen M,
et al: Long-term results of laparoscopic extended surgery in
advanced gastric cancer: a series of 101 patients.
Hepatogastroenterology. 53:304–308. 2006.PubMed/NCBI
|