Introduction
Advanced hepatocellular carcinoma (HCC) is a fatal
disease without curative measures, with a poor overall survival
(OS) of <6 months (1). Although
the management of advanced HCC has significantly changed over the
last few years due to improved patient stratification and
introduction of novel therapies, it remains debatable which
treatment should be considered as the ‘standard therapy’ for
advanced HCC cases (2). Sorafenib
is currently recommended for HCC patients with Barcelona Clinic
Liver Cancer (BCLC) stage C (3,4).
This is based on two large-scale phase III randomized controlled
trials. The Sorafenib HCC Assessment Randomized Protocol (SHARP)
trial demonstrated that sorafenib, a tyrosine kinase inhibitor, was
able to increase median survival from 7.9 to 10.7 months (5). In the corresponding Asia-Pacific
study, the effects of sorafenib in delaying time-to-progression
(TTP) and improving OS were further validated (6). However, despite the convincing data,
the two studies failed to demonstrate a statistically significant
benefit of sorafenib in patients with extrahepatic metastasis. No
statistically significant beneficial effect was observed in
patients with macroscopic vascular invasion in the Asia-Pacific
study. Furthermore, the majority of subjects enrolled in those
studies belonged to Child-Pugh (CP) class A (95% in the SHARP trial
and 97% in the Asia-Pacific study). Further studies demonstrated
that patients who received sorafenib with suboptimal liver function
(CP class B) exhibited a poorer outcome compared to those with CP
class A (7–10). As such, in BCLC stage C HCC
patients with CP class B and/or extrahepatic metastasis, sorafenib
may not be a priority choice and systemic chemotherapy remains a
viable option.
Several combinations of existing chemotherapeutic
agents have been used for phase II trials in advanced HCC; however,
only a few were able to achieve a response rate of >20%
(11). Among the regimens, the
combination of 5-fluorouracil, mitoxantrone and cisplatin (FMP)
consistently achieved a response rate of >20% in several
studies. However, despite a significant response rate, severe side
effects were reported, limiting the clinical use of FMP for
advanced HCC (11,12). In order to overcome this
difficulty, the identification of a reliable marker, capable of
predicting therapeutic responses and, thus, preventing unnecessary
side effects, is urgently required. To achieve this goal, a pilot
genome-wide association study was conducted and a group of
single-nucleotide polymorphism (SNP) markers in patients receiving
the standard FMP regimen were identified (13). It was demonstrated that the leading
marker, rs9679162, located on the intron of the
UDP-N-acetyl-α-D-galactosamine:polypeptide
N-acetylgalactosaminyltransferase 14 gene (GALNT14)
was capable of predicting the therapeutic response, but not OS, in
a previous small-scale validation study (13). To minimize the side effects, we
conducted a study using a split-dose FMP protocol, based on the
metronomic chemotherapy principle (14). The results revealed that the
hematological toxicity was significantly reduced with the
split-dose FMP regimen, without a significant alteration of the
OS.
Recently, a prospective study demonstrated that an
SNP on GALNT14 may predict the therapeutic response, as well
as OS, in patients receiving chemotherapy with split-dose FMP
(15). However, that study did not
investigate the effect of other clinical parameters, including side
effects, on the therapeutic response to split-dose FMP. In this
study, we analyzed the effect of clinical parameters,
GALNT14 genotype and side effects on TTP and OS after the
first cycle of split-dose FMP regimen. Besides the GALNT14
genotype, we identified pretreatment α-fetoprotein (AFP) levels,
on-treatment leukopenia and absence of vomiting as independent
prognostic factors. These results highlight that, with careful
selection, chemotherapy may be an optimal treatment option for a
specific group of patients with advanced HCC in BCLC stage C, with
CP class B, portal vein invasion and/or distant metastasis.
Patients and methods
Patients
This study was approved by the Institutional Review
Board of the Chang Gang Memorial Hospital, Taoyuan, Taiwan. Between
January, 2007 and December, 2012, a total of 129 patients were
diagnosed with advanced HCC in BCLC stage C, with main portal vein
thrombosis (PVT), or distant metastasis, or both. Of these
patients, 118 received at least one course of therapy and at least
one post-treatment imaging evaluation for outcome assessment. The
remaining 11 patients were excluded, due to either failure to
complete the first course of chemotherapy, inability to assess the
outcome, or refusal to sign an informed consent. The clinical
parameters recorded included gender, age, hepatitis B virus surface
antigen (HBsAg), anti-hepatitis C virus antibody (anti-HCV),
alcoholism, Eastern Cooperative Oncology Group (ECOG) performance
status (16) ascites, CP
classification, prior treatment, tumor size, PVT and distant
metastasis. Biochemistry and hemogram analysis included bilirubin,
alanine transaminase, albumin, creatinine, leukocyte count,
neutrophil percentage, hemoglobin, platelet count, prothrombin time
and AFP.
HCC was diagnosed by biopsy, aspiration cytology
and/or high AFP levels (>400 ng/ml), plus two dynamic imaging
studies (dynamic computer tomography and angiography).
Split-dose FMP regimen
The split-dose FMP regimen was modified from the
standard regimen as previously described (14). The regimen was as follows:
5-fluorouracil was administered continuously via the intravenous
route at a dose of 450 mg/m2 on days 1–5. Mitoxantrone
was administered via intravenous infusion at a dose of 3
mg/m2 on day 1. Cisplatin was administered as an
intravenous infusion at a dose of 40 mg/m2 over 2 h on
day 1 with standard hydration. On the ninth day, the biochemical
and hematological data were obtained. If the hepatic, renal and
hematological data were satisfactory, 1/4 dose of mitoxantrone and
cisplatin was administered on days 9 and 10, respectively. However,
if the data indicated severe toxicity, the 1/4 doses were further
delayed for one week, until recovery. The treatment was repeated
every 4–6 weeks until a maximum of six courses. If grade 3/4
neutropenia and/or leukopenia were observed, granulocyte
colony-stimulating factor was administered. Concomitant oral
antiviral medication for hepatitis B was allowed. None of the
patients received concomitant interferon-based therapy.
GALNT14 genotyping
Genotyping of GALNT14 was performed as
previously described (15).
Briefly, nuclear DNA was extracted and purified from the peripheral
blood prior to treatment. The primers were as follows: forward,
5′-TCACGAGGCCAACATTCTAG-3′ and reverse, 5′-TTAGATTCTGCATGGCTCAC-3′,
designed for PCR and direct sequencing of a 172-bp intronic region
of GALNT14 covering rs9679162. The SNP was determined by
sequencing data from both directions.
Survival and on-treatment side effects
evaluation
At least one lesion was measurable prior to
treatment in one dimension in all the evaluated patients. The
objective tumor response was assessed by computer tomography every
4–8 weeks after the initiation of chemotherapy. OS was calculated
from the date of treatment initiation to the date of death or last
follow-up. TTP was calculated from the date of treatment initiation
to disease progression. Based on the National Cancer Institute
Common Terminology Criteria for adverse events version 3.0
(17), on-treatment side effects
were evaluated at day 9 of FMP chemotherapy.
Statistical analysis
All the statistical analyses were performed with
SPSS version 18.0 software (SPSS, Inc., Chicago, IL, USA). The data
are presented as ratios (%) for dichotomized variables, as means ±
standard deviation for continuous variables with normal
distribution and as median (range) for continuous variables with a
non-normal distribution. For comparisons between groups, the
Chi-square or Fisher’s exact tests were used for dichotomized data,
the two-sample Student’s t-test was used for continuous variables
with normal distribution and the Mann-Whitney U test was used for
continuous variables with a non-normal distribution. The parametric
data were dichotomized into two groups, with the medians as
cut-offs. Univariate and multivariate Cox proportional hazard
models were used to estimate TTP and OS for clinical parameters,
on-treatment side effects and genotypic variables. Those variables
that were statistically significant in the univariate analysis at
the level of P<0.05 were included in the multivariate analysis.
Following categorization, the Kaplan-Meier method was used to
estimate the survival probability between groups and the log-rank
test was used to compare survival outcomes. P<0.05 was
considered to indicate a statistically significant difference.
Results
Basic clinical data of patients with
advanced HCC
A total of 118 patients were included. The basic
clinical characteristics are listed in Table I. Of the 118 patients, 26 (22.0%)
had an ECOG performance status score of ≥2. CP scoring revealed
that 115 patients (97.5%) were CP class B. The etiological analysis
demonstrated that 71 patients (60.2%) were HBsAg-positive, 42
(35.6%) were positive for anti-HCV, 8 (6.8%) had hepatitis B and C
co-infection and 13 (11.0%) had non-B and non-C etiologies.
Notably, alcoholism was observed in 37 (52.1%) of the patients with
chronic hepatitis B, 14 (33.3%) of those with hepatitis C, 4
(50.0%) of those with hepatitis B and C co-infection and in 6
(46.2%) of those with non-B and non-C etiologies, suggesting
prevalence of a combination etiology of viral infection and
alcoholism (data not shown). The GALNT14 genotype analysis
revealed that 30 patients (25.4%) had the ‘TT’ genotype. The tumor
size measured by the greatest diameter was 7.8±4.17 cm. Distant
metastasis was identified in 65 patients (55.1%), main PVT in 81
(68.6%) and both distant metastasis and PVT in 38 patients (32.2%),
reflecting an advanced stage of HCC. A total of 63 patients (53.4%)
had received previous treatment, including surgical resection,
transcatheter arterial chemoembolization and radiotherapy, whereas
none of the patients had previously received systemic chemotherapy
or targeted drugs.
 | Table IBaseline characteristics of 118
patients with advanced HCC treated by chemotherapy. |
Table I
Baseline characteristics of 118
patients with advanced HCC treated by chemotherapy.
|
Characteristics | Total series
(n=118) | Cirrhosis
(n=97) | Non-cirrhosis
(n=21) | P-value |
|---|
| Age (years) | 58.0±13.7 | 58.7±12.9 | 54.7±16.6 | 0.223 |
| Gender, male, n
(%) | 94 (79.7) | 75 (77.3) | 19 (90.5) | 0.101 |
| HBV, n (%) | 71 (60.2) | 55 (56.7) | 16 (76.2) | 0.080 |
| HCV, n (%) | 42 (35.6) | 40 (41.2) | 2 (9.5) | 0.000 |
| ECOG score
(0/1/2/3) | 59/33/25/1 | 49/26/21/1 | 10/7/4/0 | 0.929 |
| Alcoholism, n
(%) | 53 (44.9) | 43 (44.3) | 10 (47.6) | 0.786 |
| Ascites, n (%) | 60 (50.8) | 56 (57.7) | 4 (19) | 0.001 |
| Child-Pugh class
(A/B/C) | 2/115/1 | 2/94/1 | 0/21/0 | 0.929 |
| GALNT14 TT
genotype, n (%) | 30 (25.4) | 26 (26.8) | 4 (19) | 0.464 |
| Prior
treatment |
| Resection, n
(%) | 7 (5.9) | 6 (6.2) | 1 (4.8) | 0.804 |
| TACE, n (%) | 49 (41.5) | 40 (41.2) | 9 (42.9) | 0.892 |
| Radiotherapy, n
(%) | 7 (5.9) | 5 (5.2) | 2 (9.5) | 0.447 |
| Tumor status |
| Largest tumor size
(cm) | 7.8±4.17 | 7.42±4.08 | 9.56±4.24 | 0.033 |
| Portal vein
thrombosis, n (%) | 81 (68.6) | 68 (70.1) | 13 (61.9) | 0.467 |
| Extrahepatic
metastasis, n (%) | 65 (55.1) | 48 (49.5) | 17 (81) | 0.004 |
| Biochemistry and
hemogram |
| Total bilirubin
(mg/dl) | 2.11±3.38 | 2.08±2.91 | 2.23±5.13 | 0.854 |
| ALT (U/l) | 49.42±34.11 | 49.14±31.79 | 50.71±44.16 | 0.849 |
| Albumin
(g/dl) | 3.33±0.55 | 3.28±0.49 | 3.55±0.75 | 0.129 |
| Creatinine
(mg/dl) | 0.78±0.29 | 0.79±0.31 | 0.73±0.26 | 0.414 |
| Leukocyte count
(x103/ml) | 6.43±2.94 | 6.11±2.89 | 7.92±2.73 | 0.010 |
| Neutrophil
percentage (%) | 68.15±10.40 | 67.44±10.89 | 71.45±7.05 | 0.042 |
| Hemoglobin
(g/dl) | 11.71±1.93 | 11.62±1.85 | 12.15±2.27 | 0.258 |
| Platelet count
(x103/ml) | 184±121 | 168±94 | 260±189 | 0.039 |
| Prothrombin time
(sec) | 13.38±1.72 | 13.47±1.76 | 12.96±1.45 | 0.219 |
| α-fetoprotein
(ng/ml) | 2,800
(2.3–7.5×105) | 2,632
(2.3–3.8×105) | 2,955
(3–7.5×105) | 0.167 |
The presence of cirrhosis was determined by either
computed tomography or ultrasonography, plus the presence of
esophageal varices determined by endoscopy. Of the 118 patients, 97
(82.2%) were cirrhotic. Compared to the non-cirrhotic patients,
cirrhotic patients exhibited a higher frequency of HCV infection
(41.2 vs. 9.5%, P<0.001), ascites (57.7 vs. 19.0%, P=0.001),
smaller tumor size (7.42±4.08 vs. 9.56±4.24 cm, P=0.033), lower
metastasis rate (49.5 vs. 81%, P=0.004), lower leukocyte count
(6.11±2.89 vs. 7.92±2.73×103/ml, P=0.01), lower
percentage of neutrophils (67.44±10.89 vs. 71.45±7.05%, P=0.042)
and lower platelet count (168±94 vs. 260±189×103/ml,
P=0.039).
Favorable prognostic predictors for TTP
and OS prior to treatment
The clinical parameters and the GALNT14
genotype accessed prior to treatment were analyzed by univariate
analysis, followed by multivariate Cox proportional hazard analysis
(Table II). It was observed that
age (P=0.021), ECOG score (P=0.001), presence of ascites (P=0.023),
GALNT14 genotype (P=0.016), tumor size (P=0.030), total
bilirubin (P=0.039), percentage of neutrophils (P=0.018) and AFP
levels (P<0.001) were associated with TTP. Following adjustment
for the confounding factors, the multivariate analysis demonstrated
that only GALNT14 ‘TT’ genotype (P=0.035) and AFP ≤2,800
ng/ml (P=0.001) were independent predictors of a favorable TTP.
 | Table IIAnalysis of factors affecting
time-to-progression (TTP) and overall survival (OS) using data from
all the patients. |
Table II
Analysis of factors affecting
time-to-progression (TTP) and overall survival (OS) using data from
all the patients.
| | TTP | OS |
|---|
| |
|
|
|---|
| | Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|
|
|
|---|
| Parameters | n | P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value |
|---|
| Age (years) | | 0.021 | 1.442 | 0.961–2.165 | 0.077 | 0.209 | | | |
| ≤58 | 60 | | | | | | | | |
| >58 | 58 | | | | | | | | |
| Gender | | 0.614 | | | | 0.965 | | | |
| Female | 24 | | | | | | | | |
| Male | 94 | | | | | | | | |
| HBsAg | | 0.539 | | | | 0.351 | | | |
| Negative | 47 | | | | | | | | |
| Positive | 71 | | | | | | | | |
| Anti-HCV | | 0.786 | | | | 0.094 | | | |
| Negative | 76 | | | | | | | | |
| Positive | 42 | | | | | | | | |
| ECOG score | | 0.001 | 1.449 | 0.899–2.334 | 0.128 | 0.001 | 1.178 | 0.707–1.960 | 0.530 |
| 0 | 59 | | | | | | | | |
| 1,2,3 | 59 | | | | | | | | |
| Alcoholism | | 0.179 | | | | 0.145 | | | |
| No | 65 | | | | | | | | |
| Yes | 53 | | | | | | | | |
| Ascites | | 0.023 | 1.101 | 0.705–1.720 | 0.672 | 0.004 | 1.314 | 0.819–2.108 | 0.257 |
| No | 58 | | | | | | | | |
| Yes | 60 | | | | | | | | |
| Cirrhosis | | 0.698 | | | | 0.962 | | | |
| No | 21 | | | | | | | | |
| Yes | 97 | | | | | | | | |
| Child-Pugh
class | | 0.950 | | | | 0.827 | | | |
| A | 2 | | | | | | | | |
| B,C | 116 | | | | | | | | |
| GALNT14 TT
genotype | | | | | | | | | |
| No | 88 | | | | | | | | |
| Yes | 30 | 0.016 | 0.582 | 0.352–0.963 | 0.035 | 0.001 | 0.446 | 0.251–0.790 | 0.006 |
| Prior
treatment | | | | | | | | | |
| Resection | | 0.573 | | | | 0.272 | | | |
| No | 111 | | | | | | | | |
| Yes | 7 | | | | | | | | |
| TACE | | 0.265 | | | | 0.133 | | | |
| No | 69 | | | | | | | | |
| Yes | 49 | | | | | | | | |
| Radiotherapy | | 0.116 | | | | 0.463 | | | |
| No | 111 | | | | | | | | |
| Yes | 7 | | | | | | | | |
| Tumor status | | | | | | | | | |
| Size (cm) | | 0.030 | 1.014 | 0.966–1.065 | 0.562 | 0.004 | 1.430 | 0.859–2.380 | 0.170 |
| ≤8 | 63 | | | | | | | | |
| >8 | 55 | | | | | | | | |
| Portal vein
thrombosis | | 0.522 | | | | 0.227 | | | |
| No | 37 | | | | | | | | |
| Yes | 81 | | | | | | | | |
| Metastasis | | 0.115 | | | | 0.417 | | | |
| No | 53 | | | | | | | | |
| Yes | 65 | | | | | | | | |
| Biochemistry and
hemogram | | | | | | | | | |
| Total bilirubin
(mg/dl) | | 0.039 | 1.203 | 0.783–1.847 | 0.399 | 0.008 | 0.928 | 0.539–1.596 | 0.787 |
| ≤1.3 | 63 | | | | | | | | |
| >1.3 | 55 | | | | | | | | |
| ALT (U/l) | | 0.193 | | | | 0.717 | | | |
| ≤39.5 | 59 | | | | | | | | |
| >39.5 | 59 | | | | | | | | |
| Albumin
(g/dl) | | 0.305 | | | | 0.004 | 0.791 | 0.474–1.320 | 0.370 |
| ≤3.3 | 65 | | | | | | | | |
| >3.3 | 53 | | | | | | | | |
| Creatinine
(mg/dl) | | 0.522 | | | | 0.916 | | | |
| ≤0.75 | 59 | | | | | | | | |
| >0.75 | 59 | | | | | | | | |
| Leukocyte count
(x103/ml) | | 0.121 | | | | 0.028 | 1.074 | 0.633–1.822 | 0.791 |
| ≤5.9 | 61 | | | | | | | | |
| >5.9 | 57 | | | | | | | | |
| Neutrophil count
(%) | | 0.018 | 0.997 | 0.633–1.569 | 0.988 | 0.001 | 1.107 | 0.638–1.919 | 0.718 |
| ≤68.95 | 59 | | | | | | | | |
| >68.95 | 59 | | | | | | | | |
| Hemoglobin
(g/dl) | | 0.281 | | | | 0.235 | | | |
| ≤11.3 | 61 | | | | | | | | |
| >11.3 | 57 | | | | | | | | |
| Platelet count
(x103/ml) | | 0.052 | | | | 0.058 | | | |
| ≤160 | 60 | | | | | | | | |
| >160 | 58 | | | | | | | | |
| Prothrombin time
(sec) | | 0.063 | | | | 0.006 | 1.560 | 0.928–2.624 | 0.094 |
| ≤13 | 59 | | | | | | | | |
| >13 | 59 | | | | | | | | |
| AFP (ng/ml) | | <0.001 | 2.019 | 1.318–3.094 | 0.001 | <0.001 | 1.706 | 1.059–2.747 | 0.028 |
| ≤2,800 | 59 | | | | | | | | |
| >2,800 | 59 | | | | | | | | |
The univariate analysis revealed that several
factors were associated with OS, including ECOG score (P=0.001),
presence of ascites (P=0.004), GALNT14 genotype (P=0.001),
tumor size (P=0.004), total bilirubin (P=0.008), albumin (P=0.004),
leukocyte count (P=0.028), percentage of neutrophils (P=0.001),
prothrombin time (P=0.006) and AFP levels (P<0.001). On
multivariate analysis, the GALNT14 genotype (P=0.006) and
AFP levels (P=0.028) were identified as independent predictors of
OS.
Association between outcome and
on-treatment side effects
The on-treatment side effects in our study were
leukopenia (55.1%), neutropenia (39.8%), anemia (85.6%),
thrombocytopenia (58.5%), nausea (55.9%), vomiting (27.1%),
mucositis (22.9%), diarrhea (24.6%), alopecia (2.5%), hepatoxicity
(16.1%), skin rash (6.8%), fatigue (77.1%), renal insufficiency
(5.9%), bleeding (12.7%) and infection (14.4%). Univariate followed
by multivariate Cox proportional hazard analysis was performed to
elucidate the association of on-treatment side effects with TTP and
OS (Table III). The favorable
factors associated with TTP were found to be leukopenia (P=0.008),
absence of vomiting (P=0.009) and absence of skin rash (P=0.023).
As regards OS, on-treatment leukopenia (P=0.027), absence of
vomiting (P=0.013), absence of skin rash (P=0.001) and absence of
renal insufficiency (P=0.030) were identified as favorable
factors.
 | Table IIIAnalysis of side effects affecting
time-to-progression (TTP) and overall survival (OS). |
Table III
Analysis of side effects affecting
time-to-progression (TTP) and overall survival (OS).
| | TTP | OS |
|---|
| |
|
|
|---|
| | Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|
|
|
|---|
| Side effects | n | P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value |
|---|
| Leukopenia | | 0.005 | 0.586 | 0.395–0.868 | 0.008 | 0.001 | 0.517 | 0.288–0.929 | 0.027 |
| No | 53 | | | | | | | | |
| Yes | 65 | | | | | | | | |
| Neutropenia | | 0.066 | | | | 0.017 | 1.003 | 0.545–1.847 | 0.991 |
| No | 71 | | | | | | | | |
| Yes | 47 | | | | | | | | |
| Anemia | | 0.617 | | | | 0.501 | | | |
| No | 17 | | | | | | | | |
| Yes | 101 | | | | | | | | |
|
Thrombocytopenia | | 0.233 | | | | 0.258 | | | |
| No | 49 | | | | | | | | |
| Yes | 69 | | | | | | | | |
| Nausea | | 0.314 | | | | 0.354 | | | |
| No | 52 | | | | | | | | |
| Yes | 66 | | | | | | | | |
| Vomiting | | 0.005 | 1.771 | 1.157–2.712 | 0.009 | 0.016 | 1.807 | 1.136–2.876 | 0.013 |
| No | 86 | | | | | | | | |
| Yes | 32 | | | | | | | | |
| Mucositis | | 0.686 | | | | 0.665 | | | |
| No | 91 | | | | | | | | |
| Yes | 27 | | | | | | | | |
| Diarrhea | | 0.924 | | | | 0.527 | | | |
| No | 89 | | | | | | | | |
| Yes | 29 | | | | | | | | |
| Alopecia | | 0.514 | | | | 0.378 | | | |
| No | 115 | | | | | | | | |
| Yes | 3 | | | | | | | | |
| Hepatoxicity | | 0.600 | | | | 0.150 | | | |
| No | 99 | | | | | | | | |
| Yes | 19 | | | | | | | | |
| Skin rash | | 0.035 | 2.334 | 1.125–4.844 | 0.023 | 0.002 | 3.489 | 1.653–7.362 | 0.001 |
| No | 110 | | | | | | | | |
| Yes | 8 | | | | | | | | |
| Fatigue | | 0.100 | | | | 0.068 | | | |
| No | 27 | | | | | | | | |
| Yes | 91 | | | | | | | | |
| Renal
insufficiency | | 0.088 | | | | 0.028 | 2.636 | 1.100–6.316 | 0.030 |
| No | 111 | | | | | | | | |
| Yes | 7 | | | | | | | | |
| Bleeding | | 0.961 | | | | 0.386 | | | |
| No | 103 | | | | | | | | |
| Yes | 15 | | | | | | | | |
| Infection | | 0.440 | | | | 0.355 | | | |
| No | 101 | | | | | | | | |
| Yes | 17 | | | | | | | | |
Identification of a subgroup of HCC
patients most suitable for FMP therapy
To identify a subgroup of patients with advanced HCC
with better TTP and OS, pretreatment AFP levels, GALNT14
genotype, on-treatment leukopenia, vomiting and skin rash were
selected for further multivariate Cox proportional hazard analysis
(Table IV). The favorable factors
associated with TTP and OS were identified as AFP levels (both
P<0.001), GALANT14 genotype (P=0.019 and 0.006,
respectively), on-treatment leukopenia (P=0.007 and 0.009,
respectively) and absence of vomiting (P=0.017 and 0.015,
respectively). Four favorable factors were analyzed using the
Kaplan-Meier survival method and the log-rank test was used to
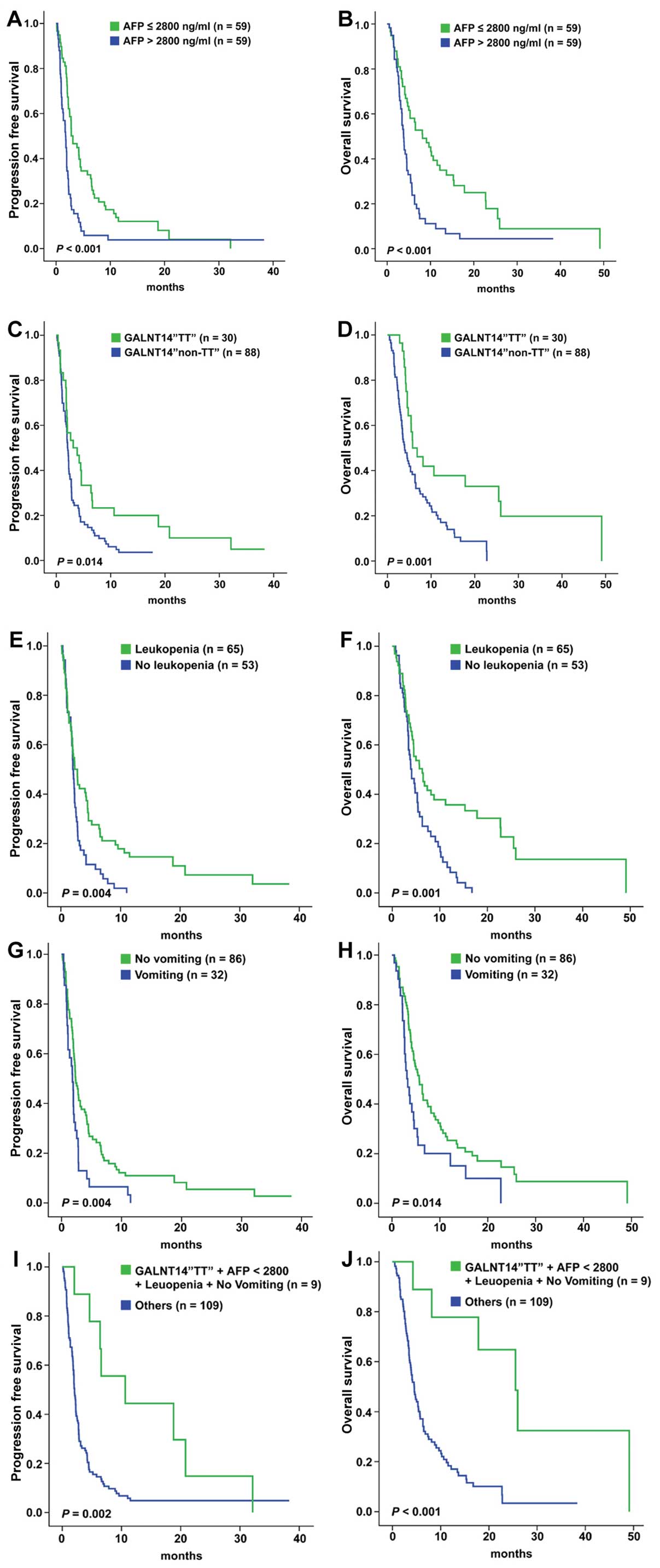
compare the TTP and OS (Fig. 1). A
total of 59 patients with AFP ≤2,800 ng/ml had longer median TTP
and OS (3.11 vs. 1.75 months, P<0.001; and 8.14 vs. 3.79 months,
P<0.001, respectively). A total of 30 patients with the
GALNT14 ‘TT’ genotype had longer median TTP and OS (3.11 vs.
2.11 months, P=0.014; and 5.75 vs. 3.93 months, P=0.001,
respectively). A total of 65 patients with leukopenia had longer
median TTP and OS (2.75 vs. 2.00 months, P=0.004; and 6.32 vs. 4.07
months, P=0.001, respectively). A total of 86 patients without
vomiting had longer median TTP and OS (2.32 vs. 1.82 months,
P=0.004; and 5.71 vs. 3.29 months, P=0.014, respectively). Finally,
9 patients (9/118; 7.6%) with all four favorable factors exhibited
the longest median TTP and OS (10.64 vs. 2.07 months, P=0.002; and
25.50 vs. 4.50 months, P<0.001, respectively).
 | Table IVMultivariate analysis of factors
affecting time-to-progression (TTP) and overall survival (OS). |
Table IV
Multivariate analysis of factors
affecting time-to-progression (TTP) and overall survival (OS).
| | TTP | OS |
|---|
| |
|
|
|---|
| Factors | n | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| AFP (ng/ml) | | 2.300 | 1.508–3.508 | <0.001 | 2.305 | 1.475–3.602 | <0.001 |
| ≤2,800 | 59 | | | | | | |
| >2,800 | 59 | | | | | | |
| GALNT14 TT
genotype | | 0.564 | 0.349–0.911 | 0.019 | 0.464 | 0.268–0.803 | 0.006 |
| No | 88 | | | | | | |
| Yes | 30 | | | | | | |
| Leukopenia | | 0.577 | 0.388–0.859 | 0.007 | 0.564 | 0.368–0.864 | 0.009 |
| No | 53 | | | | | | |
| Yes | 65 | | | | | | |
| Vomiting | | 1.679 | 1.098–2.569 | 0.017 | 1.772 | 1.118–2.807 | 0.015 |
| No | 86 | | | | | | |
| Yes | 32 | | | | | | |
| Skin rash | | 1.236 | 0.570–2.681 | 0.591 | 1.748 | 0.805–3.798 | 0.158 |
| No | 110 | | | | | | |
| Yes | 8 | | | | | | |
Since the GALNT14 genotype was identified as
a favorable prognostic factor, we further performed Kaplan-Meier
survival analysis for GALNT14 ‘TT’ genotype patients with
either three of the newly identified factors, or all factors. Among
the 30 patients with the GALNT14 ‘TT’ genotype, 13 with AFP
levels ≤2,800 ng/ml had longer median TTP and OS (6.51 vs. 1.82
months, P=0.049; and 17.82 vs. 5.39 months, P=0.044, respectively).
Of the patients with on-treatment leukopenia, 20 had a longer
median OS (17.82 vs. 4.75 months, P=0.007), but not TTP (4.61 vs.
2.00 months, P=0.056). Of the patients without on-treatment
vomiting, 24 had a longer median TTP (3.86 vs. 1.82 months,
P=0.041), but not OS (8.14 vs. 4.14 months, P=0.070) (data not
shown).
A total of 9 patients with the GALNT14 ‘TT’
genotype and three additional favorable factors exhibited the
longest median TTP and OS (10.64 vs. 1.96 months, P=0.024; and
25.50 vs. 4.71 months, P=0.018, respectively) (data not shown).
Since pretreatment AFP level as a prognostic factor
is easy to obtain in clinical practice, 59 patients with AFP ≤2,800
ng/ml were further analyzed with the Kaplan-Meier survival method
using other prognostic factors. A total of 13 patients with the
GALNT14 ‘TT’ genotype had longer median TTP and OS (6.57 vs.
2.75 months, P=0.017; and 17.82 vs. 6.50 months, P=0.005,
respectively). A total of 33 patients with on-treatment leukopenia
had longer median TTP and OS (4.46 vs. 2.50 months, P=0.008; and
17.82 vs. 5.28 months, P=0.001, respectively). A total of 44
patients without on-treatment vomiting had a longer median TTP
(4.21 vs. 2.07 months, P=0.017), but not OS (9.64 vs. 4.61 months,
P=0.088). Finally, 9 patients with AFP ≤2,800 ng/ml and three
additional favorable factors exhibited the longest median TTP and
OS (10.64 vs. 2.79 months, P=0.005; and 25.50 vs. 6.50 months,
P=0.001, respectively) (data not shown).
Discussion
Current guidelines (3,4)
recommend sorafenib as the first-line treatment for advanced HCC,
based on the results of two phase III randomized controlled trials
(5,6). However, not all advanced-stage HCC
patients benefit from sorafenib. Those two studies failed to
demonstrate statistically significant benefits in patients with
extrahepatic spread or poor liver function. Furthermore, a
retrospective study on HCC patients treated with soranefib
demonstrated a trend towards worse OS from CP class A to B: the
median OS was 6.1, 5.4 and 2.7 months in CP class A, CP class B
(score 7) and CP class B patients (score 8 and 9), respectively
(8). In the subset of patients
with CP class B, the optimal treatment has not been clearly
determined. Chemotherapy has been considered as a therapeutic
option in patients with advanced HCC; however, the severe side
effects and lack of prognostic predictors limit its clinical use.
Our group previously demonstrated that the split-dose FMP regimen
achieved a similar OS compared to the standard FMP (5.2 vs. 6.0
months, P=0.447), but was associated with a significantly lower
risk of severe neutropenia (5.1 vs. 10.5%, P=0.0005) (14). A pilot genome-wide association
study and a prospective confirmatory study further verified the
predictive value of the GALNT14 genotype (13,15).
For advanced HCC patients with CP class B receiving spit-dose FMP
therapy, in those with GALNT14 ‘TT’ vs. those with the
‘non-TT’ genotype, the median TTP was 3.9 vs. 2.1 months,
respectively (P<0.001) and the OS was 6.8 vs. 3.9 months,
respectively (P<0.001).
In this study, we retrospectively investigated 118
patients with advanced HCC receiving split-dose FMP chemotherapy,
97.5% of whom were CP class B. Besides the GALNT14 ‘TT’
genotype, we further identified three favorable predictors of
outcome, including AFP ≤2,800 ng/ml, on-treatment leukopenia and
absence of vomiting after the first course of split-dose FMP. By
simply using the AFP level, a group with better outcome (median
TTP, 3.11 months; OS, 8.14 months) was identified. With the
combination of GALNT14 ‘TT’ genotype, an even better median
TTP (6.51 months) and OS (17.82 months) may be achieved.
Furthermore, on-treatment side effects, including leukopenia and
vomiting, may help predict the prognosis. Patients with four
favorable factors exhibited the longest median TTP (10.64 months)
and OS (25.5 months). Our study provided strong evidence that
split-dose FMP chemotherapy may be considered as an effective
treatment in patients with advanced HCC with AFP ≤2,800 ng/ml
and/or the GALNT14 ‘TT’ genotype.
The serum AFP level is a useful marker used for HCC
screening and diagnosis worldwide. Although AFP is not elevated in
all patients with HCC, a high serum AFP has been associated with
advanced tumor stage, including greater tumor size, bilobar
involvement, massive or diffuse-type tumor, poorer differentiation
and PVT (18,19). The serum AFP level was found to be
significantly higher in HCC patients with BCLC stage D compared to
stage A and B (18) and was also
associated with prognosis. In addition, the AFP level has been
considered as an important predictor of postoperative HCC
recurrence and metastasis (20,21).
In patients with advanced HCC treated with either sorafenib,
transarterial chemoembolization, hepatic artery infusional
chemotherapy or concurrent chemoradiotherapy, an early reduction of
AFP was found to be a predictor of positive outcome (22–25).
Furthermore, the early elevation of AFP was shown to be a predictor
of unfavorable outcome in patients with advanced HCC treated with
sorafenib (26). In addition, it
was demonstrated that a low serum AFP level (≤50 ng/ml) was a
favorable predictor for patients treated with intravenous
5-fluorouracil and subcutaneous recombinant interferon-α-2b
(27), suggesting a predictive
value of AFP in patients receiving chemotherapy. In this study, the
patients with BCLC stage C HCC were divided into two groups by the
median level of AFP (2,800 ng/ml). We demonstrated that patients
with AFP ≤2,800 ng/ml treated with split-dose FMP regimen had a
significantly better prognosis compared to those with AFP >2,800
ng/ml. This result may be explained in part by the fact that
ephrin-A1 expression (an angiogenic factor) in HCC was shown to
increase AFP levels and the ability of AFP to elicit the escape of
HCC cells from immune surveillance (28,29).
However, whether the AFP level is directly associated with
chemoresistance of tumor cells remains unclear.
The severe side effects of chemotherapy, such as
leukopenia/neutropenia, have been considered as one of the major
obstacles for its clinical use. However, it was also demonstrated
that there is an association between chemotherapy-induced
myelotoxicity and patient outcome in a number of malignancies,
including lung, breast, gastric, ovarian and colorectal cancer
(30–38). A meta-analysis of 13 trials on
various types of cancers (n=9,528) demonstrated a 31% reduction in
the mortality risk for patients with a higher grade of neutropenia
or leukopenia, compared to patients with lower-grade or no
cytopenia (39). Our findings were
consistent with those of previous studies on other malignancies,
demonstrating that on-treatment leukopenia is also a prognostic
factor in patients with advanced HCC treated with split-dose FMP
chemotherapy. It was hypothesized that leukopenia/neutropenia, an
indicator of bone marrow suppression caused by a particular dose of
a chemotherapeutic agent, may also be a surrogate marker indicating
that the same dose is adequate to provide an anticancer effect.
Thus, lack of leukopenia or neutropenia may indicate an
insufficient or absence of biological effect of chemotherapy.
Of note, the absence of vomiting was also identified
as a favorable predictor in this study. Although this information
may help predict outcome, the underlying mechanisms have not been
elucidated. The presence of vomiting in patients with advanced HCC
may be induced by extra-abdominal factors (chemotherapeutic agents,
electrolyte abnormalities, central nervous system involvement) or
intra-abdominal factors (gastroparesis, ileus, gastric outlet
obstruction, bowel obstruction) (40). We hypothesized that on-treatment
vomiting indicates poor enteral nutrition in patients with advanced
HCC, which may lead to cachexia and compromise the response to
antineoplastic therapy.
This study had several notable limitations,
including its retrospective nature, limited sampling and
restriction of the sample to a Chinese population. Our eligibility
criteria confined the treatment regimen to split-dose FMP for
interpretative clarity. Therefore, it may not pertain to other
chemotherapeutic regimens. It is also unclear whether the leukocyte
count measured at day 9 of chemotherapy actually represents the
nadir in each patient. However, despite these drawbacks, our study
is considered to be suitable for routine clinical practice.
In conclusion, it was recently demonstrated that
ideal therapeutic results were not achieved by sorafenib in
patients with advanced HCC with CP class B (9). In such patients, we identified a
subgroup exhibiting a better clinical outcome when treated with the
split-dose FMP chemotherapeutic regimen, simply by assessing the
AFP level and/or the GALNT14 genotype. The performance may
be further improved by including on-treatment leukopenia and
absence of vomiting as predictors. In patients with 4 favorable
prognostic factors, a median TTP of >10 months and an OS of
>25 months may be expected.
Acknowledgements
We would like to thank the staff members of the
Liver Research Center for their technical assistance. This study
was partly funded by grants from the National Science Council,
Taiwan (no. NMRPD1B0052), the Chang Gung University, Taiwan (no.
SCRPD1C0071) and the Chang Gung Memorial Hospital, Taiwan (no.
CLRPG3C0011).
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
ALT
|
alanine transaminase
|
|
AST
|
aspartate transaminase
|
|
BCLC
|
Barcelona Clinic Liver Cancer
|
|
CP
|
Child-Pugh
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
FMP
|
5-fluorouracil, mitoxantrone and
cisplatin
|
|
GALNT14
|
UDP-N-acetyl-α-D-galactosamine:polypeptide
N-acetylgalactosaminyltransferase 14
|
|
HBsAg
|
hepatitis B virus surface antigen
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
OS
|
overall survival
|
|
PVT
|
portal vein thrombosis
|
|
SHARP
|
Sorafenib HCC Assessment Randomized
Protocol
|
|
SNP
|
single-nucleotide polymorphism
|
|
TACE
|
transcatheter arterial
chemoembolization
|
|
TTP
|
time-to-progression
|
References
|
1
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: the BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A, Hernandez-Gea V and Llovet
JM: Medical therapies for hepatocellular carcinoma: a critical view
of the evidence. Nat Rev Gastroenterol Hepatol. 10:34–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases. Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
European Association for the Study of the
Liver; European Organisation for Research and Treatment of Cancer.
EASL-EORTC clinical practice guidelines: management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012.PubMed/NCBI
|
|
5
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar
|
|
7
|
Abou-Alfa GK, Amadori D, Santoro A, et al:
Safety and efficacy of sorafenib in patients with hepatocellular
carcinoma (HCC) and Child-Pugh A versus B cirrhosis. Gastrointest
Cancer Res. 4:40–44. 2011.PubMed/NCBI
|
|
8
|
Chiu J, Tang YF, Yao TJ, et al: The use of
single-agent sorafenib in the treatment of advanced hepatocellular
carcinoma patients with underlying Child-Pugh B liver cirrhosis: a
retrospective analysis of efficacy, safety, and survival benefits.
Cancer. 118:5293–5301. 2012. View Article : Google Scholar
|
|
9
|
Kim HY, Park JW, Joo J, et al: Worse
outcome of sorafenib therapy associated with ascites and Child-Pugh
score in advanced hepatocellular carcinoma. J Gastroenterol
Hepatol. 28:1756–1761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pressiani T, Boni C, Rimassa L, et al:
Sorafenib in patients with Child-Pugh class A and B advanced
hepatocellular carcinoma: a prospective feasibility analysis. Ann
Oncol. 24:406–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu AX: Systemic therapy of advanced
hepatocellular carcinoma: how hopeful should we be? Oncologist.
11:790–800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang TS, Chang HK, Chen JS, Lin YC, Liau
CT and Chang WC: Chemotherapy using 5-fluorouracil, mitoxantrone,
and cisplatin for patients with advanced hepatocellular carcinoma:
an analysis of 63 cases. J Gastroenterol. 39:362–369. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang KH, Lin CC and Yeh CT: GALNT14 SNP
as a potential predictor of response to combination chemotherapy
using 5-FU, mitoxantrone and cisplatin in advanced HCC.
Pharmacogenomics. 12:1061–1073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeh CT, Chen HC, Sung CM, et al:
Retrospective comparison between a regular and a split-dose
protocol of 5-fluorouracil, cisplatin, and mitoxantrone for the
treatment of far advanced hepatocellular carcinoma. BMC Cancer.
11:1172011. View Article : Google Scholar
|
|
15
|
Yeh CT, Liang KH, Lin CC, Chang ML, Hsu CL
and Hung CF: A single nucleotide polymorphism on the GALNT14 gene
as an effective predictor of response to chemotherapy in advanced
hepatocellular carcinoma. Int J Cancer. 134:1214–1224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Xiao GQ, Yan LN, et al: Value of
α-fetoprotein in association with clinicopathological features of
hepatocellular carcinoma. World J Gastroenterol. 19:1811–1819.
2013.
|
|
19
|
Tangkijvanich P1, Anukulkarnkusol N,
Suwangool P, et al: Clinical characteristics and prognosis of
hepatocellular carcinoma: analysis based on serum alpha-fetoprotein
levels. J Clin Gastroenterol. 31:302–308. 2000. View Article : Google Scholar
|
|
20
|
Ma WJ, Wang HY and Teng LS: Correlation
analysis of preoperative serum alpha-fetoprotein (AFP) level and
prognosis of hepatocellular carcinoma (HCC) after hepatectomy.
World J Surg Oncol. 11:2122013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang SK, Hlaing WW, Yu RQ, Lee TW,
Ganpathi IS and Madhavan KK: Value of alpha-foetoprotein for
screening of recurrence in hepatocellular carcinoma post resection.
Singapore Med J. 53:32–35. 2012.PubMed/NCBI
|
|
22
|
Lee YK, Kim SU, Kim do Y, et al:
Prognostic value of α-fetoprotein and des-γ-carboxy prothrombin
responses in patients with hepatocellular carcinoma treated with
transarterial chemoembolization. BMC Cancer. 13:52013.
|
|
23
|
Personeni N, Bozzarelli S, Pressiani T, et
al: Usefulness of alpha-fetoprotein response in patients treated
with sorafenib for advanced hepatocellular carcinoma. J Hepatol.
57:101–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Memon K, Kulik L, Lewandowski RJ, et al:
Alpha-fetoprotein response correlates with EASL response and
survival in solitary hepatocellular carcinoma treated with
transarterial therapies: a subgroup analysis. J Hepatol.
56:1112–1120. 2012. View Article : Google Scholar
|
|
25
|
Lee MH, Kim SU, Kim do Y, et al: Early
on-treatment predictions of clinical outcomes using
alpha-fetoprotein and des-gamma-carboxy prothrombin responses in
patients with advanced hepatocellular carcinoma. J Gastroenterol
Hepatol. 27:313–322. 2012. View Article : Google Scholar
|
|
26
|
Nakazawa T, Hidaka H, Takada J, et al:
Early increase in α-fetoprotein for predicting unfavorable clinical
outcomes in patients with advanced hepatocellular carcinoma treated
with sorafenib. Eur J Gastroenterol Hepatol. 25:683–689. 2013.
|
|
27
|
Patt YZ, Yoffe B, Charnsangavej C, et al:
Low serum alpha-fetoprotein level in patients with hepatocellular
carcinoma as a predictor of response to 5-FU and
interferon-alpha-2b. Cancer. 72:2574–2582. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li MS, Ma QL, Chen Q, et al:
Alpha-fetoprotein triggers hepatoma cells escaping from immune
surveillance through altering the expression of Fas/FasL and tumor
necrosis factor related apoptosis-inducing ligand and its receptor
of lymphocytes and liver cancer cells. World J Gastroenterol.
11:2564–2569. 2005. View Article : Google Scholar
|
|
29
|
Iida H, Honda M, Kawai HF, et al:
Ephrin-A1 expression contributes to the malignant characteristics
of {alpha}-fetoprotein producing hepatocellular carcinoma. Gut.
54:843–851. 2005.PubMed/NCBI
|
|
30
|
Lee CK, Simes RJ, Brown C, et al:
Prognostic nomogram to predict progression-free survival in
patients with platinum-sensitive recurrent ovarian cancer. Br J
Cancer. 105:1144–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shitara K, Matsuo K, Takahari D, et al:
Neutropenia as a prognostic factor in advanced gastric cancer
patients undergoing second-line chemotherapy with weekly
paclitaxel. Ann Oncol. 21:2403–2409. 2010. View Article : Google Scholar
|
|
32
|
Shitara K, Matsuo K, Takahari D, et al:
Neutropaenia as a prognostic factor in metastatic colorectal cancer
patients undergoing chemotherapy with first-line FOLFOX. Eur J
Cancer. 45:1757–1763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pallis AG, Agelaki S, Kakolyris S, et al:
Chemotherapy-induced neutropenia as a prognostic factor in patients
with advanced non-small cell lung cancer treated with front-line
docetaxel-gemcitabine chemotherapy. Lung Cancer. 62:356–363. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koutras AK, Fountzilas G, Dafni U, et al;
Hellenic Cooperative Oncology Group. Myelotoxicity as a prognostic
factor in patients with advanced breast cancer treated with
chemotherapy: a pooled analysis of two randomised trials conducted
by the Hellenic Cooperative Oncology Group. Anticancer Res.
28:2913–2920. 2008.
|
|
35
|
Yamanaka T, Matsumoto S, Teramukai S,
Ishiwata R, Nagai Y and Fukushima M: Predictive value of
chemotherapy-induced neutropenia for the efficacy of oral
fluoropyrimidine S-1 in advanced gastric carcinoma. Br J Cancer.
97:37–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Di Maio M, Gridelli C, Gallo C, et al:
Chemotherapy-induced neutropenia and treatment efficacy in advanced
non-small-cell lung cancer: a pooled analysis of three randomised
trials. Lancet Oncol. 6:669–677. 2005.PubMed/NCBI
|
|
37
|
Poikonen P, Saarto T, Lundin J, Joensuu H
and Blomqvist C: Leucocyte nadir as a marker for chemotherapy
efficacy in node-positive breast cancer treated with adjuvant CMF.
Br J Cancer. 80:1763–1766. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saarto T, Blomqvist C, Rissanen P, Auvinen
A and Elomaa I: Haematological toxicity: a marker of adjuvant
chemotherapy efficacy in stage II and III breast cancer. Br J
Cancer. 75:301–305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shitara K, Matsuo K, Oze I, et al:
Meta-analysis of neutropenia or leukopenia as a prognostic factor
in patients with malignant disease undergoing chemotherapy. Cancer
Chemother Pharmacol. 68:301–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lagman RL, Davis MP, LeGrand SB and Walsh
D: Common symptoms in advanced cancer. Surg Clin North Am.
85:237–255. 2005. View Article : Google Scholar
|