Introduction
Esophageal carcinoma is one of the most aggressive
malignancies worldwide, with a poor prognosis and a mortality rate
approximating 100/105 in China (1). The 2 subtypes of esophageal
carcinoma, esophageal squamous cell carcinoma (ESCC) and esophageal
adenocarcinoma (EAC), exhibit different incidences among different
countries. EAC is currently rapidly increasing in incidence in the
United States; however, ESCC remains the predominant histological
type in Eastern countries, particularly China (2–4).
Surgery remains the curative treatment option for patients with
non-metastatic ESCC. However, ≥60% of the patients are unsuitable
for surgery due to the advanced disease stage and the presence of
comorbidities (5). Therefore,
chemotherapy remains one of the main therapeutic options. Achieving
an optimal therapeutic effect with chemotherapy is crucial in the
treatment of esophageal cancer.
Ginsenoside Rg3, the active ingredient extracted
from Panax ginseng, possesses anticancer properties and
exerts various pharmacological effects (6,7).
Previous studies demonstrated that ginsenoside Rg3 may inhibit
cancer growth in vitro and in vivo and is considered
to be relatively safe (8–10). However, the molecular mechanisms
underlying the effects of ginsenoside Rg3 have been not yet been
fully elucidated. Ginsenoside Rg3 was reported to reduce tumor
proliferation, angiogenesis and metastasis. Ginsenoside Rg3 may be
a beneficial supplement, enhancing the inhibitory effects of
chemotherapy.
This study used a ESCC xenograft mouse model to
evaluate the tumor inhibitory effect of ginsenoside Rg3 combined
with chemotherapy.
Materials and methods
Cell culture
Eca-109 human esophageal squamous carcinoma cells
were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). The Eca-109 cells were cultured in
RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd, Hangzhou, China) and 100 μg/ml streptomycin
(NCPC, Shijiazhuang, China) and they were kept in an incubator
containing 5% CO2 at 37°C.
Animals
Female BALB/c nude mice, weighing 18–20 g and aged
5–6 weeks, were purchased from Beijing HFK Bioscience Co., Ltd.,
(Beijing, China). All the mice were raised under specified
pathogen-free conditions (22±1C, 12-h light/dark cycle) and fed
with standard chow diet and tap water. The study experiments were
performed in accordance with the guidelines approved by the
Laboratory Animal Care Committee of Hebei Province.
Experimental design
The Eca-109 cells were diluted in phosphate-buffered
saline to form a single-cell suspension. The right forelimbs of the
BALB/c nude mice were subcutaneously injected with 5×106
cells (0.1 ml serum-free medium). When the diameter of tumors
reached 7 mm, the mice were randomly assigned to 4 treatment groups
(n=5 per group), namely the control group (saline treatment), the
ginsenoside Rg3 alone, the chemotherapy alone and the chemotherapy
+ ginsenoside Rg3 groups. The mice in the control group were
intraperitoneally injected with 0.2 ml 0.9% saline for 21 days. The
ginsenoside Rg3 group was treated with 0.2 ml ginsenoside Rg3
(purity, 96.1%; Zhejiang Yatai Pharmaceutical Co., Ltd., Shenzhen,
Zhejiang, China) at 6 mg/kg/day by gavage administration once daily
for 3 weeks. The chemotherapy group was administered a combination
regimen (paclitaxel 10 mg/kg/day + cisplatin 5 mg/kg/day)
intraperitoneally on days 1, 7, 14 and 21. Cisplatin was provided
by World House Pharmaceuticals Co., Ltd., (Jiangsu, China) and
paclitaxel was obtained from Ha Medicine Group (Harbin, China). The
chemotherapy + ginsenoside Rg3 group received the same chemotherapy
regimen combined with ginsenoside Rg3. Tumor size (length and
width) was measured using calipers every other day and the tumor
volumes (cm3) were estimated using the formula 0.52 ×
length × width2. During the experimental period, the
weight loss and any change in the drinking and/or eating habits of
the mice was observed and recorded.
Immunohistochemistry and microvascular
density (MVD) evaluation
After the xenograft mice were sacrificed by cervical
dislocation, the tumor tissues were excised, weighed and fixed
immediately in neutral formalin, then used for hematoxylin and
eosin staining and immunohistochemical assays. Rabbit anti-human
Ki-67 antibodies were used (dilution, 1:100; Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China). Ki-67 protein
expression was detected with a SABC kit according to the
manufacturer’s instructions (Booster Bioengineering Institute,
Wuhan, China). To evaluate MVD, the sections were examined under a
light microscope (BX41; Olympus, Tokyo, Japan) to identify 3
regions with the highest MVD. The microvessels were counted in
these areas (magnification, ×400) and the number of microvessels
was recorded. The average number was defined as the MVD of the
tumor.
Statistical analysis
The data are expressed as means ± standard
deviation. The Student’s t-test and one-way analysis of variance
were used to assess the statistical significance of the differences
between treatment groups. Data analyses were performed with
Statistical Analysis System V8 (SAS Institute Inc., Cary, NC, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Animals
During the treatment period, no mice died in any of
the groups until the experiment was completed. Furthermore, the
weight of the mice was not significantly reduced in any of the 4
groups and there were no significant treatment-related adverse
effects.
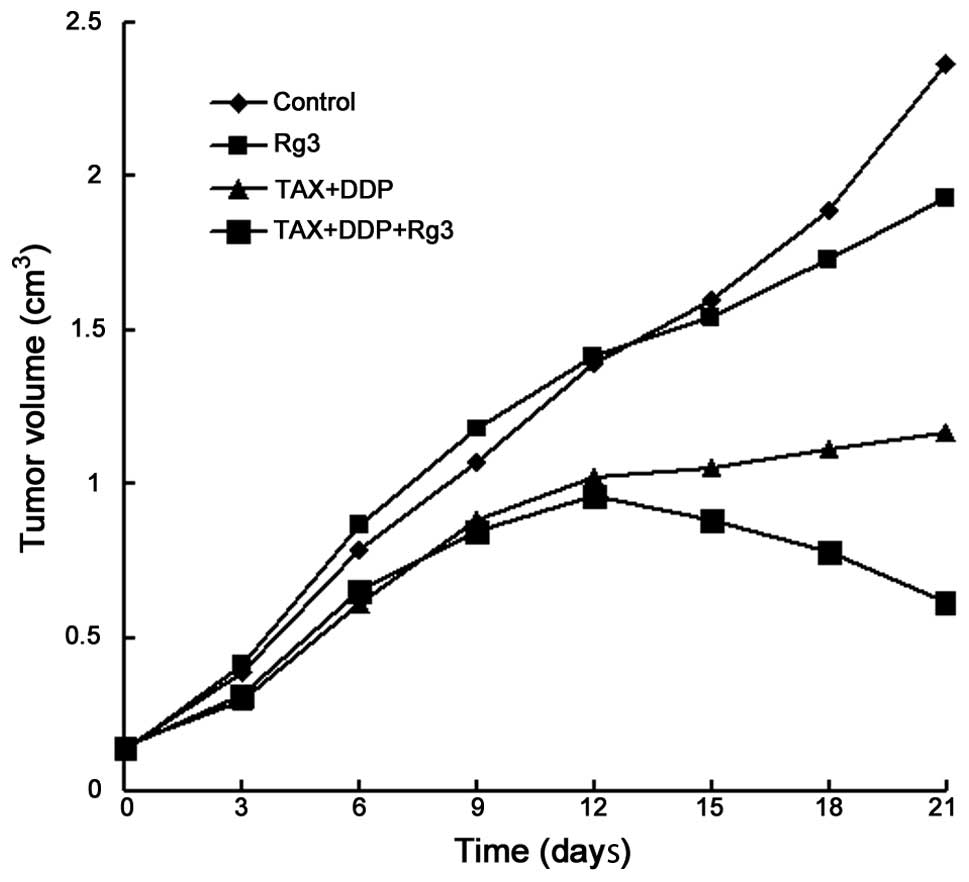
Tumor size and inhibitory rate
The tumor volumes (cm3) at different time
points following treatment are shown in Table I. Prior to administration (day 0),
the mean tumor volume did not differ significantly among the
control, ginsenoside Rg3, chemotherapy and chemotherapy +
ginsenoside Rg3 groups (0.136±0.008, 0.127±0.020, 0.147±0.018 and
0.142±0.016 cm3, respectively; P=0.2511). We observed
that the tumor volume in the chemotherapy + ginsenoside Rg3 group
at 15 days began to exhibit a significant decrease compared to the
other 3 groups (P<0.0001). In the ginsenoside Rg3 group, the
tumor volume was significantly lower compared to that in the
control group at 18 days (P<0.0001), but higher compared to that
in the chemotherapy alone and chemotherapy + ginsenoside Rg3
groups. As shown in Fig. 1, the
growth curve of the tumor in different groups was drawn according
to the mean tumor volumes. The inhibitory rates of the tumor in
each group are listed in Table
II. On day 21, the inhibitory rate of the tumor in the
ginsenoside Rg3, chemotherapy and chemotherapy + ginsenoside Rg3
groups was 24.31, 59.67 and 70.64%, respectively.
 | Table ITumor volumes (cm3) at
different time points following treatment. |
Table I
Tumor volumes (cm3) at
different time points following treatment.
| | Groups | |
|---|
| |
| |
|---|
| Time points | No. | Control | Rg3 | TAX+DDP | TAX+DDP+Rg3 | P-value |
|---|
| Day 0 | 5 | 0.136±0.008 | 0.127±0.020 | 0.147±0.018 | 0.142±0.016 | 0.2511 |
| Day 3 | 5 | 0.383±0.018 | 0.406±0.015a | 0.291±0.018a,b | 0.308±0.017a,b | <0.0001 |
| Day 6 | 5 | 0.799±0.092 | 0.863±0.076 | 0.609±0.028a,b | 0.648±0.149a,b | 0.0019 |
| Day 9 | 5 | 1.067±0.083 | 1.177±0.076 | 0.882±0.107a,b | 0.845±0.121a,b | 0.0002 |
| Day 12 | 5 | 1.390±0.101 | 1.416±0.100 | 1.018±0.102a,b | 0.959±0.126a,b | <0.0001 |
| Day 15 | 5 | 1.597±0.101 | 1.541±0.103 | 1.050±0.113a,b | 0.878±0.108a,b,c | <0.0001 |
| Day 18 | 5 | 1.890±0.137 | 1.729±0.110a | 1.108±0.115a,b | 0.774±0.102a,b,c | <0.0001 |
| Day 21 | 5 | 2.367±0.134 | 1.931±0.107a | 1.165±0.123a,b | 0.614±0.090a,b,c | <0.0001 |
 | Table IITumor weight and inhibitory rate of
tumor in different groups. |
Table II
Tumor weight and inhibitory rate of
tumor in different groups.
| Groups | No. | Tumor weight (g) | Inhibitory rate
(%) |
|---|
| Control | 5 | 1.847±0.111 | - |
| Rg3 | 5 | 1.398±0.236a | 24.31 |
| TAX+DDP | 5 | 0.745±0.082a | 59.67 |
| TAX+DDP+Rg3 | 5 | 0.542±0.099a | 70.64 |
Staining characteristics
The tumor tissue specimens were stained with
hematoxylin and eosin for the histopathological assay and the
histopathological changes were examined under a light microscope.
We observed that the chemotherapy + ginsenoside Rg3 group exhibited
small, shadow-stained nuclei compared to the other groups (Fig. 2A). In addition, the staining for
the proliferation marker Ki-67 in the chemotherapy + ginsenoside
Rg3 group was less prominent compared to that in the other groups
(Fig. 2B).
CD34 immunohistochemical staining was used to assess
the microvasculature of the tumor. As shown in Fig. 3, the MVD was statistically
significantly different among groups (P<0.05). The chemotherapy
+ ginsenoside Rg3 group exhibited the lowest MVD among the groups
(P<0.05). These results demonstrated that the combination of
ginsenoside Rg3 with chemotherapy exerted significant inhibitory
effects on Eca-109 ESCC in mice.
Discussion
Ginseng has been a widely recognized traditional
medicine in Eastern Asian countries for thousands of years and is
becoming increasingly popular in Western countries. Accumulating
studies suggest that the main anticancer ingredient of ginseng is
ginsenoside Rg3 (11). A number of
studies clearly demonstrated that ginsenoside Rg3 inhibits cancer
growth in vivo and in vitro through reducing tumor
proliferation, angiogenesis and metastasis (12–14).
Ginsenoside Rg3 combined with capecitabine enhanced
antiangiogenic efficacy in breast cancer in mice and exhibited
improved antitumor effects and reduced toxicity (15). In addition, ginsenoside Rg3 was
shown to inhibit CXCR4 expression and related migrations in the
MDA-MB-231 breast cancer cell line (16). Rg3 promoted the efficacy of
cisplatin by inhibiting heme oxygenase 1 and NAD(P)H dehydrogenase
(quinone 1) expression in CT-26 colon cancer cells and protected
the kidney and liver against tissue damage by preventing
cisplatin-induced intracellular reactive oxygen species generation
(17). In addition, ginsenoside
Rg3 enhanced the susceptibility of SW620 and HCT116 colon cancer
cells to docetaxel and other chemotherapeutic agents via nuclear
factor κ-light-chain-enhancer of activated B cells (NF-κB)
inhibition (18). Jiang et
al (19) reported that
ginsenoside Rg3 inhibited hepatocellular carcinoma growth via the
intrinsic apoptotic pathway. Moreover, ginsenoside Rg3 combined
with gemcitabine was found to significantly inhibit angiogenesis
and growth of lung cancer and improve survival and quality of life
in tumor-bearing mice (20). Pan
et al (21) reported that
ginsenoside Rg3 attenuates cell migration via inhibition of
aquaporin 1 expression in PC-3M prostate cancer cells. Furthermore,
Kim et al (22) confirmed
that the combination of ginsenoside Rg3 with docetaxel enhanced the
susceptibility of prostate cancer cells via inhibition of NF-κB.
However, it had not been elucidated whether ginsenoside Rg3
combined with paclitaxel and cisplatin enhances their inhibitory
effects on the Eca-109 xenograft in mice.
In our study, ginsenoside Rg3 in combination with
chemotherapy significantly inhibited the growth of the Eca-109
xenograft in nude mice. After 3 weeks of treatment, the inhibitory
rate in the ginsenoside Rg3 + chemotherapy group reached 70.64% and
was significantly higher compared to that in the other treatment
groups. In addition, the combination group exhibited a lower Ki-67
expression compared to that in the other 3 groups and the lowest
MVD among all groups. In conclusion, ginsenoside Rg3 improved the
antitumor efficacy of chemotherapy in Eca-109 ESCC in mice.
References
|
1
|
Liu M, Zhang F, Liu S, et al:
Microsatellite analysis in multistage carcinogenesis of esophageal
squamous cell carcinoma from Chongqing in Southern China. Int J Mol
Sci. 12:7401–7409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berg D, Wolff C, Langer R, et al:
Discovery of new molecular subtypes in oesophageal adenocarcinoma.
PLoS One. 6. pp. e239852011, View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang Y, Hou X, Cui Q, et al: Skp2
expression unfavorably impacts survival in resectable esophageal
squamous cell carcinoma. J Transl Med. 10:732012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Etemadi A, Abnet CC, Golozar A, et al:
Modeling the risk of esophageal squamous cell carcinoma and
squamous dysplasia in a high risk area in Iran. Arch Iran Med.
15:18–21. 2012.PubMed/NCBI
|
|
5
|
Li R, Chen TW, Wang LY, et al:
Quantitative measurement of contrast enhancement of esophageal
squamous cell carcinoma on clinical MDCT. World J Radiol.
4:179–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joo EJ, Ha YW, Shin H, et al: Generation
and characterization of monoclonal antibody to ginsenoside rg3.
Biol Pharm Bull. 32:548–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shinkai K, Akedo H, Mukai M, et al:
Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Jpn
J Cancer Res. 87:357–362. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo X, Wang CZ, Chen J, et al:
Characterization of gene expression regulated by American ginseng
and ginsenoside Rg3 in human colorectal cancer cells. Int J Oncol.
32:975–983. 2008.PubMed/NCBI
|
|
9
|
Kim K, Park M and Young Kim H: Ginsenoside
Rg3 suppresses palmitate-induced apoptosis in MIN6N8 pancreatic
beta-cells. J Clin Biochem Nutr. 46:30–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SY, Kim GT, Roh SH, et al: Proteomic
analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human
colon cancer cell lines. Biosci Biotechnol Biochem. 73:811–816.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dougherty U, Mustafi R, Wang Y, et al:
American ginseng suppresses Western diet-promoted tumorigenesis in
model of inflammation-associated colon cancer: role of EGFR. BMC
Complement Altern Med. 11:1112011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang CZ, Aung HH, Zhang B, et al:
Chemopreventive effects of heat-processed Panax
quinquefolius root on human breast cancer cells. Anticancer
Res. 28:2545–2551. 2008.PubMed/NCBI
|
|
13
|
Zhang C, Liu L, Yu Y, et al: Antitumor
effects of ginsenoside Rg3 on human hepatocellular carcinoma cells.
Mol Med Rep. 5:1295–1298. 2012.PubMed/NCBI
|
|
14
|
Yuan HD, Quan HY, Zhang Y, et al:
20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells
is associated with AMPK signaling pathway. Mol Med Rep. 3:825–831.
2010.PubMed/NCBI
|
|
15
|
Zhang Q, Kang X, Yang B, et al:
Antiangiogenic effect of capecitabine combined with ginsenoside Rg3
on breast cancer in mice. Cancer Biother Radiopharm. 23:647–653.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen XP, Qian LL, Jiang H and Chen JH:
Ginsenoside Rg3 inhibits CXCR4 expression and related migrations in
a breast cancer cell line. Int J Clin Oncol. 16:519–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CK, Park KK, Chung AS and Chung WY:
Ginsenoside Rg3 enhances the chemosensitivity of tumors to
cisplatin by reducing the basal level of nuclear factor erythroid
2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone
oxidoreductase-1 and prevents normal tissue damage by scavenging
cisplatin-induced intracellular reactive oxygen species. Food Chem
Toxicol. 50:2565–2574. 2012.
|
|
18
|
Kim SM, Lee SY, Yuk DY, et al: Inhibition
of NF-kappaB by ginsenoside Rg3 enhances the susceptibility of
colon cancer cells to docetaxel. Arch Pharm Res. 32:755–765. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang JW, Chen XM, Chen XH and Zheng SS:
Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via
intrinsic apoptotic pathway. World J Gastroenterol. 17:3605–3613.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu TG, Huang Y, Cui DD, et al: Inhibitory
effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis
and growth of lung cancer in mice. BMC Cancer. 9:2502009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan XY, Guo H, Han J, et al: Ginsenoside
Rg3 attenuates cell migration via inhibition of aquaporin 1
expression in PC-3M prostate cancer cells. Eur J Pharmacol.
683:27–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SM, Lee SY, Cho JS, et al: Combination
of ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol.
631:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|