Introduction
Primary liver cancer (PLC) is the fifth most common
malignancy worldwide (1) and
hepatocellular carcinoma (HCC) is the most common histological type
of PLC (2). HCC commonly develops
in patients with carcinogenetic backgrounds of chronic viral
infection, such as hepatitis B virus (HBV) or hepatitis C virus, or
alcoholic liver injury. Primary liver sarcomatoid carcinoma (SC) is
a rare tumor that may be associated with HCC and cholangiocarcinoma
(2). Primary liver SC may also
arise as a pure sarcomatous carcinoma, which is associated with a
poor prognosis due to rapid growth and high recurrence rate
following resection (3). This is
the case report of a patient with combined HCC and primary liver
SC, in a background of HBV chronic viral hepatitis with cirrhosis
and alcoholic liver injury. To the best of our knowledge, this is
the first case report of combined HCC and primary liver SC.
Case report
A 54-year-old man, who had been routinely checked
for chronic hepatitis associated with HBV infection, liver cirhosis
and alcoholic liver injury, complained of fatigue and general
weakness. The biochemical indices of liver function were
unremarkable. Abdominal ultrasonography revealed mild hepatic
shrinkage and coarse nodular echoes. There was a small enhancing
nodule in S5–S6 and a small hypoechoic nodule in the right lobe of
the liver. Abdominal computed tomography (CT) and magnetic
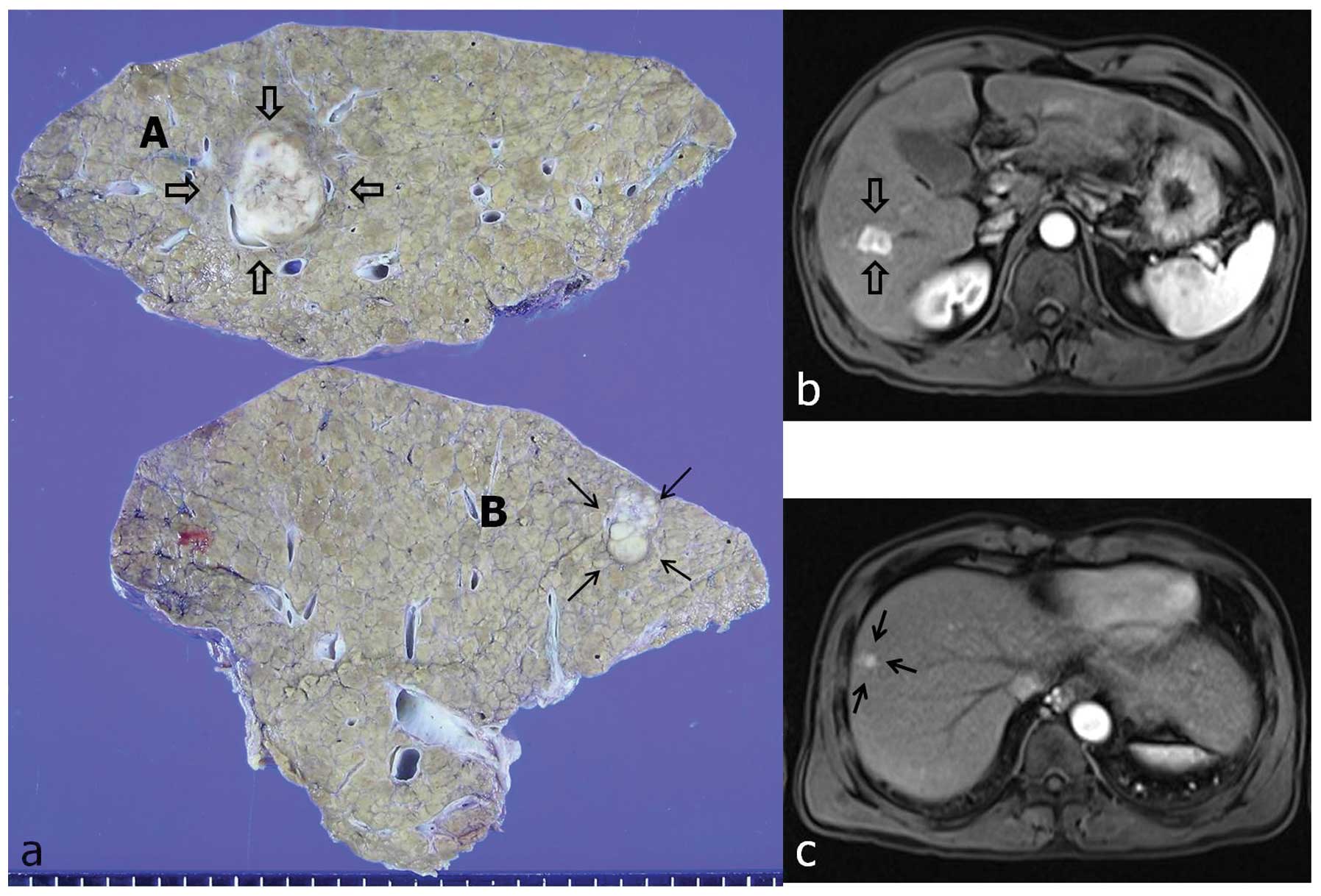
resonance imaging (MRI) revealed two masses in the right robe of
the liver, one in S5–S6, sized 2 cm (Fig. 1b) and the other in S8, sized 1.3 cm
(Fig. 1c). Right lobectomy was
performed upon a clinical diagnosis of double HCC. Grossly, the
surgical specimen displayed two distinct masses, in a background of
typical multinodular cirrhotic changes. The larger mass was
well-demarcated, measuring 2.5×2.0 cm, of gray to white color and
exhibiting central hemorrhage (Fig.
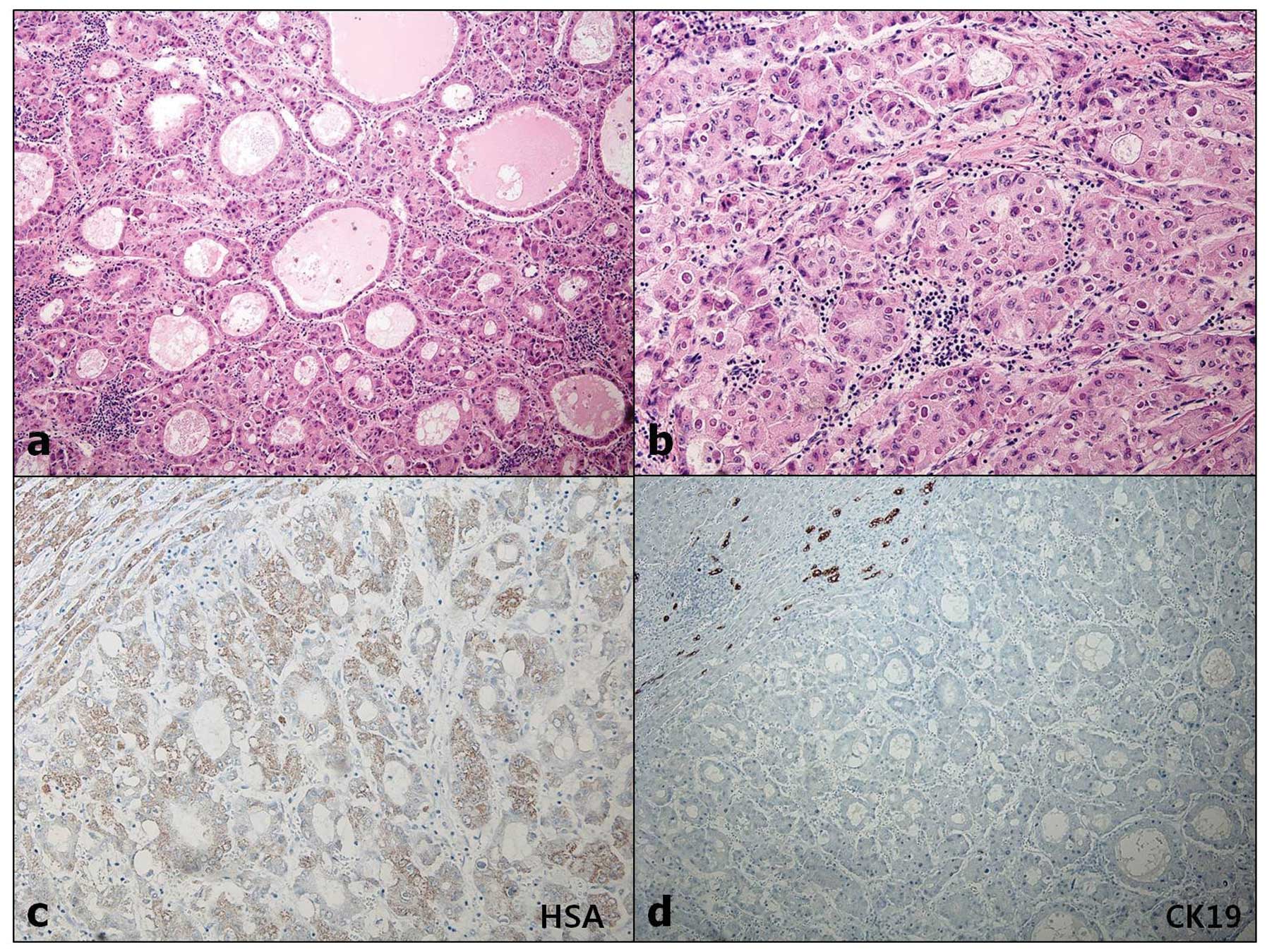
1a, labeled as ‘A’). Microscopically, the tumor was composed of
pleomorphic cells of round to oval and spindle shape, haphazardly
arranged, with frequent atypical mitotic figures. The microscopic
characteristics were not suggestive of HCC (Fig. 2a). The tumor cells were
immunoreactive for cytokeratin (CK) (Fig. 2b) and vimentin (VMT) (Fig. 2c), but negative for
hepatocyte-specific antigen (HSA) (Fig. 2d), CK19, CK20 and CD68. The other
mass was a white to gray nodule, sized 1.3×1.0 cm, with small
satellite lesions (Fig. 1a,
labeled as ‘B’). Microscopically, variable characteristics of HCC
were observed, with a pseudoglandular and trabecular appearance
(Fig. 3a and b). The tumor cells
were positive for HSA (Fig. 3c)
and CK, but negative for CK7, CK19 and VMT (Fig. 3d). There was no transition or
intermingling of cells between the two tumors. The final diagnosis
was double primary tumor of the liver (primary liver SC and HCC).
At 4 months after resection, the patient developed uncontrolled
ascites and succumbed to peritoneal cancer seeding.
Discussion
Primary liver SC and carcinosarcoma (CS) of the
liver comprise a mixture of carcinomatous and sarcomatous elements
and are very rare worldwide, with only a few such cases reported in
the English literature (4). CS is
defined by the World Health Organization (WHO) as a malignant tumor
that consists of an intimate mixture of carcinomatous (either HCC
or cholangiocellular carcinoma) and sarcomatous elements (5). If the sarcomatous areas with
malignant epithelial components were composed of variable malignant
mesenchymal components, the tumor would be considered to be a CS.
However, if the sarcomatous areas were composed of only malignant
spindle cells and were shown to display epithelial characteristics
based on immunohistochemical and electron microscopic findings, the
tumor would be diagnosed as SC or spindle cell carcinoma (6). The sarcomatous elements of CS lack
epithelial markers and the tumor is considered a true heterogonous
sarcoma, whereas the sarcomatous element of SC retains the
expression of epithelial markers (4). In the present case, the morphological
characteristics of the tumor were similar to those of
undifferentiated sarcoma. The microscopic examination of
hematoxylin-eosin stained specimens revealed a diffuse
proliferation of atypical and pleomorphic spindle cells with
hyperchromatic nuclei, high nuclear-to-cytoplasmic ratio and poor
cellular adhesion. Frequent atypical mitotic figures were also
identified. Immunohistochemically, the tumor cells were
immunoreactive for epithelial markers (CK).
The pathogenesis of the sarcomatoid component in
primary SC and CS of the liver remains unclear. Therefore,
knowledge of the histogenesis of sarcomatoid transformation in
liver cancer is crucial (4).
Murata et al (7) reported
that genetic and immunohistochemical analyses support the
hypothesis that undifferentiated, sarcomatoid HCC and
cholangiocarcinoma may be derived from an original HCC, while
Fayyazi et al hypothesized that this tumor arises from
totipotent stem cells that are able to differentiate into both
epithelial and mesenchymal cells (8), and other researchers suggested that
the carcinomatous components were likely to transform into
sarcomatous components through a metaplastic process (9,10).
Certain authors reported that the neoplastic cells of conventional
HCC may be capable of transforming into multipotent immature cells,
which, in turn, redifferentiate into sarcomatous components
(9,11–14).
In addition, the WHO tumor classification (4th edition) suggests
that the sarcomatoid component represents clonal evolution from a
differentiated component (HCC or cholangiocarcinoma) (15).
In addition to SC, there was another tumor in this
patient, which was diagnosed as HCC with a pseudoglandular and
trabecular pattern. To the best of our knowledge, this is the first
case report of synchronous development of hepatic SC and HCC,
whereas on preoperative radiological studies (abdominal ultrasound,
CT and MRI) these two masses were considered to be HCCs, due to
their similar imaging characteristics.
We reported a case of synchronously detected primary
liver SC and HCC in a background of HBV chronic viral hepatitis
with cirrhosis and alcoholic liver injury. In conclusion, as
primary liver SC is an aggressive tumor with a high recurrence rate
and a tendency for rapid growth, accurate and prompt diagnosis
through thorough tissue sampling and intensive immunohistochemical
analysis is crucial.
Acknowledgements
This study was supported by research funds from the
Chosun University, Republic of Korea, 2013.
References
|
1
|
Cao J, Huang L, Liu C, et al: Double
primary hepatic cancer (hepatocellular carcinoma and intrahepatic
cholangiocarcinoma) in a single patient: a clinicopathologic study
of 35 resected cases. J Gastroenterol Hepatol. 28:1025–1031. 2013.
View Article : Google Scholar
|
|
2
|
Uemoto J, Hoshi N, Hirabayashi K, et al:
Collision tumors of hepatocellular carcinoma and malignant
peritoneal mesothelioma. Med Mol Morphol. 46:177–183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yokomizo J, Cho A, Yamamoto H, et al:
Sarcomatous hepatocellular carcinoma without previous anticancer
therapy. J Hepatobiliary Pancreat Surg. 14:324–327. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang QB, Cui BK, Weng JM, Wu QL, Qiu JL
and Lin X: Clinicopathological characteristics and outcome of
primary sarcomatoid carcinoma and carcinosarcoma of the liver. J
Gastrointest Surg. 16:1715–1726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nomura K, Aizawa S and Ushigome S:
Carcinosarcoma of the liver. Arch Pathol Lab Med. 124:888–890.
2000.PubMed/NCBI
|
|
6
|
Kwon JH, Kang YN and Kang KJ:
Carcinosarcoma of the liver: a case report. Korean J Radiol.
8:343–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murata M, Miyoshi Y, Iwao K, et al:
Combined hepatocellular/cholangiocellular carcinoma with
sarcomatoid features: genetic analysis for histogenesis. Hepatol
Res. 21:220–227. 2001. View Article : Google Scholar
|
|
8
|
Fayyazi A, Nolte W, Oestmann JW, Sattler
B, Ramadori G and Radzun HJ: Carcinosarcoma of the liver.
Histopatholology. 32:385–387. 1998. View Article : Google Scholar
|
|
9
|
Kubosawa H, Ishige H, Kondo Y, Konno A,
Yamamoto T and Nagao K: Hepatocellular carcinoma with
rhabdomyoblastic differentiation. Cancer. 62:781–786. 1988.
View Article : Google Scholar
|
|
10
|
Rosai J: Rosai and Ackerman’s Surgical
Pathology. 9th edition. Mosby; Edinburgh: pp. 2592004
|
|
11
|
Lao XM, Chen DY, Zhang YQ, Xiang J, Guo
RP, Lin XJ and Li JQ: Primary carcinosarcoma of the liver:
clinicopathologic features of 5 cases and a review of the
literature. Am J Surg Pathol. 31:817–826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maeda T, Adachi E, Kajiyama K, Takenaka K,
Sugimachi K and Tsuneyoshi M: Spindle cell hepatocellular
carcinoma. A clinicopathologic and immunohistochemical analysis of
15 cases. Cancer. 77:51–57. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wick MR and Swanson PE: Carcinosarcomas:
current perspectives and an historical review of nosological
concepts. Semin Diagn Pathol. 10:118–127. 1993.PubMed/NCBI
|
|
14
|
Nakajima T, Kubosawa H, Kondo Y, Konno A
and Iwama S: Combined hepatocellular-cholangiocarcinoma with
variable sarcomatous transformation. Am J Clin Pathol. 90:309–312.
1998.PubMed/NCBI
|
|
15
|
Miettinen M, Fletcher CDM, Kindblom LG,
Zimmermann A and Tsui WMS: Mesenchymal tumours of the liver. World
Health Organization Classification of Tumours of the Digestive
System. Bosman FT, carneiro F, Hruban RH, Theise ND, Fred T and
Bosman: 4th edition. IARC Press; Lyon: pp. 2492010
|