Introduction
Gastric cancer is one of the most common causes of
cancer-related mortality. One million new cases are diagnosed
annually, accounting for 700,000 mortalities worldwide (1, 2).
Undifferentiated gastric carcinomas are generally associated with a
worse prognosis (3). Mucinous
gastric carcinoma (MGC) is a rare histological subtype of
undifferentiated gastric carcinoma, accounting for 2.6–6.6% of all
gastric cancer cases (4–8). The available literature on MGC is
currently limited, mostly due to its rarity. Several previous
studies have suggested that the prognosis of MGC patients is poor
(5, 9, 10),
whereas others reported no differences in characteristics and
prognosis between MGC and non-MGC (NMGC) cases (7, 11).
Thus, the clinicopathological characteristics and prognosis of MGC
following surgical resection remain controversial. The present
study aimed to determine the clinicopathological characteristics
and postoperative survival of MGC patients.
Materials and methods
Patients
We identified 2,706 patients who underwent surgical
treatment for gastric cancer between 1990 and 2010 at the
Department of Surgery, Kurume University School of Medicine
(Fukuoka, Japan). Patients with gastric cancer in the residual
stomach following a prior gastrectomy and those undergoing surgery
after an endoscopic procedure were excluded. Microscopic
examination of hematoxylin and eosin-stained tissue sections from
formalin-fixed, paraffin-embedded surgical specimens revealed 70
cases of MGC and 2,492 of NMGC. MGC was defined by the World Health
Organization as an adenocarcinoma, in which over half of the tumor
area contained extracellular mucin pools (12).
The study design and procedures were in accordance
with the Declaration of Helsinki and were approved by the Ethics
Committee of Kurume University (no. 14057). All the participants
provided written informed consent.
Clinicopathological
characteristics
We retrospectively reviewed the patients' medical
charts, surgical records and histopathological reports to collect
information on their clinicopathological characteristics, including
age, gender, tumor size, tumor location, macroscopic type,
histological type, depth of invasion, lymph node metastasis,
lymphovascular invasion, distant metastasis and tumor stage. The
tumor characteristics were defined according to the Japanese
Classification of Gastric Carcinoma (3rd English edition) (13). All the patients were regularly
followed up according to our standard protocol (at least every 3
months for 5 years), which included tumor marker studies,
gastrointestinal endoscopy, ultrasonography and computed
tomography.
Statistical analyses
The clinicopathological factors were compared using
the Fisher's exact test or the Pearson's χ2 test, as
appropriate. Disease-specific survival rates were analyzed using
the Kaplan-Meier method and comparisons between groups were
assessed by the log-rank test. In the multivariate analysis, the
Cox proportional hazards model was used to identify independent
prognostic factors. A P-value of <0.05 was considered to
indicate statistically significant differences. All the statistical
analyses were performed using JMP 10 software (SAS Institute, Inc.,
Cary, NC, USA).
Results
Clinicopathological
characteristics
Table I summarizes
the characteristics of all 2,562 patients included in the present
study. Of the 70 MGC cases (2.7% of all resected gastric cancer
cases in this study), only 6 (8.6%) were early-stage, whereas the
remaining 64 patients (91.4%) had advanced-stage disease. When
compared to NMGC tumors, MGC tumors were larger in size (83.0 vs.
54.9 mm), were more frequently Borrmann type 2 and 3 (70.0 vs.
28.5%), presented with a higher rate of T4 invasion of the gastric
wall (72.9 vs. 32.5%), positive N2 and N3 lymph node metastasis
(62.9 vs. 25.5%), positive lymphatic vessel invasion (100.0 vs.
63.0%), positive venous invasion (72.9 vs. 39.6%), peritoneal
metastasis (24.3 vs. 6.1%) and advanced tumor stages III and IV
(67.1 vs. 29.4%). The clinicopathological characteristics of stage
III and IV MGC and NMGC were also compared (Table II), revealing significant
differences only in the peritoneal and hepatic metastasis status.
MGC patients experienced a significantly higher incidence of
peritoneal metastasis compared to NMGC patients (36.2 vs. 20.9%,
respectively; P=0.014), whereas hepatic metastasis was more
frequently encountered in NMGC patients (0.0 vs. 10.3%;
P=0.021).
 | Table IComparison of clinicopathological
characteristics between MGC and NMGC patients. |
Table I
Comparison of clinicopathological
characteristics between MGC and NMGC patients.
| MGC (n=70) | NMGC (n=2,492) | |
|---|
|
|
| |
|---|
| Characteristics | No. | % | No. | % | P-value |
|---|
| Age, years (mean ±
SD) | 66.8±11.0 | 65.0±11.4 | 0.207 |
| Gender | | | | | 0.583 |
| Male | 50 |
71.4 | 1,703 |
68.3 | |
|
Female | 20 |
28.6 | 789 |
31.7 | |
| Tumor size, mm (mean
± SD) | 83.0±41.4 | 54.9±39.7 |
<0.001a |
| Tumor location | | | | |
0.345 |
|
Upper | 12 |
17.1 | 486 |
19.5 | |
|
Middle | 16 |
22.9 | 738 |
29.6 | |
|
Lower | 33 |
47.1 | 1,063 |
42.7 | |
|
Whole | 9 |
12.9 | 205 |
8.2 | |
| Macroscopic type | | | | |
<0.001a |
| Borrmann
0 | 7 |
10.0 | 1,377 |
55.3 | |
| Borrmann
1 | 5 |
7.1 | 52 |
2.1 | |
| Borrmann
2 | 18 |
25.7 | 294 |
11.8 | |
| Borrmann
3 | 31 |
44.3 | 416 |
16.7 | |
| Borrmann
4 | 4 |
5.8 | 173 |
6.9 | |
| Borrmann
5 | 5 |
7.1 | 180 |
7.2 | |
| Depth of
invasion | | | | |
<0.001a |
| T1 | 6 |
8.6 | 1,373 |
55.1 | |
| T2 | 8 |
11.4 | 191 |
7.7 | |
| T3 | 5 |
7.1 | 117 |
4.7 | |
| T4 | 51 |
72.9 | 811 |
32.5 | |
| Lymph node
metastasis | | | | |
<0.001a |
| N0 | 19 |
27.1 | 1,610 |
64.6 | |
| N1 | 7 |
10.0 | 246 |
9.9 | |
| N2 | 13 |
18.6 | 220 |
8.8 | |
| N3 | 31 |
44.3 | 416 |
16.7 | |
| Lymphatic
invasion | 70 |
100.0 | 1,569 |
63.0 |
<0.001a |
| Venous invasion | 51 |
72.9 | 986 |
39.6 |
<0.001a |
| Peritoneal
metastasis | 17 |
24.3 | 153 |
6.1 |
<0.001a |
| Hepatic
metastasis | 0 |
0.0 | 75 |
3.0 |
0.141 |
| Stage | | | | |
<0.001a |
| I | 9 |
12.9 | 1,448 |
58.1 | |
| II | 14 |
20.0 | 312 |
12.5 | |
|
III | 19 |
27.1 | 388 |
15.6 | |
| IV | 28 |
40.0 | 344 |
13.8 |
 | Table IIComparison of clinicopathological
characteristics between stage III and IV MGC and NMGC patients. |
Table II
Comparison of clinicopathological
characteristics between stage III and IV MGC and NMGC patients.
| MGC (n=47) | NMGC (n=732) | |
|---|
|
|
| |
|---|
|
Characteristics | No. | % | No. | % | P-value |
|---|
| Age, years (mean ±
SD) | 65.4±11.4 | 65.5±11.4 |
0.986 |
| Gender | | | | |
0.912 |
|
Male | 32 |
68.1 | 504 |
68.9 | |
|
Female | 15 |
31.9 | 228 |
31.1 | |
| Tumor size, mm
(mean ± SD) | 98.6±39.6 | 92.5±41.0 |
0.343 |
| Tumor location | | | | |
0.153 |
|
Upper | 7 |
14.9 | 182 |
24.9 | |
|
Middle | 7 |
14.9 | 121 |
16.5 | |
|
Lower | 24 |
51.1 | 258 |
35.2 | |
|
Whole | 9 |
19.1 | 171 |
23.4 | |
| Macroscopic
type | | | | |
0.364 |
|
Borrmann 0 | 1 |
2.1 | 6 |
0.8 | |
|
Borrmann 1 | 1 |
2.1 | 24 |
3.3 | |
|
Borrmann 2 | 13 |
27.7 | 171 |
23.3 | |
|
Borrmann 3 | 25 |
53.2 | 324 |
44.3 | |
|
Borrmann 4 | 4 |
8.5 | 149 |
20.4 | |
|
Borrmann 5 | 3 |
6.4 | 58 |
7.9 |
| Depth of
invasion | | | | |
0.928 |
| T1 | 0 |
0.0 | 5 |
0.7 | |
| T2 | 1 |
2.1 | 21 |
2.9 | |
| T3 | 3 |
6.4 | 41 |
5.6 | |
| T4 | 43 |
91.5 | 665 |
90.8 | |
| Lymph node
metastasis | | | | |
0.411 |
| N0 | 1 |
2.1 | 26 |
3.5 | |
| N1 | 4 |
8.5 | 122 |
16.7 | |
| N2 | 11 |
23.4 | 175 |
23.9 | |
| N3 | 31 |
66.0 | 409 |
55.9 | |
| Lymphatic
invasion | 47 |
100.0 | 731 |
99.9 |
0.800 |
| Venous
invasion | 41 |
87.2 | 656 |
89.6 |
0.606 |
| Peritoneal
metastasis | 17 |
36.2 | 153 |
20.9 | 0.014a |
| Hepatic
metastasis | 0 |
0.0 | 75 |
10.3 | 0.021a |
| Stage | | | | |
0.094 |
|
III | 19 |
40.4 | 388 |
53.0 | |
| IV | 28 |
59.6 | 344 |
47.0 | |
Postoperative survival
The median follow-up period was 61.0 months (range,
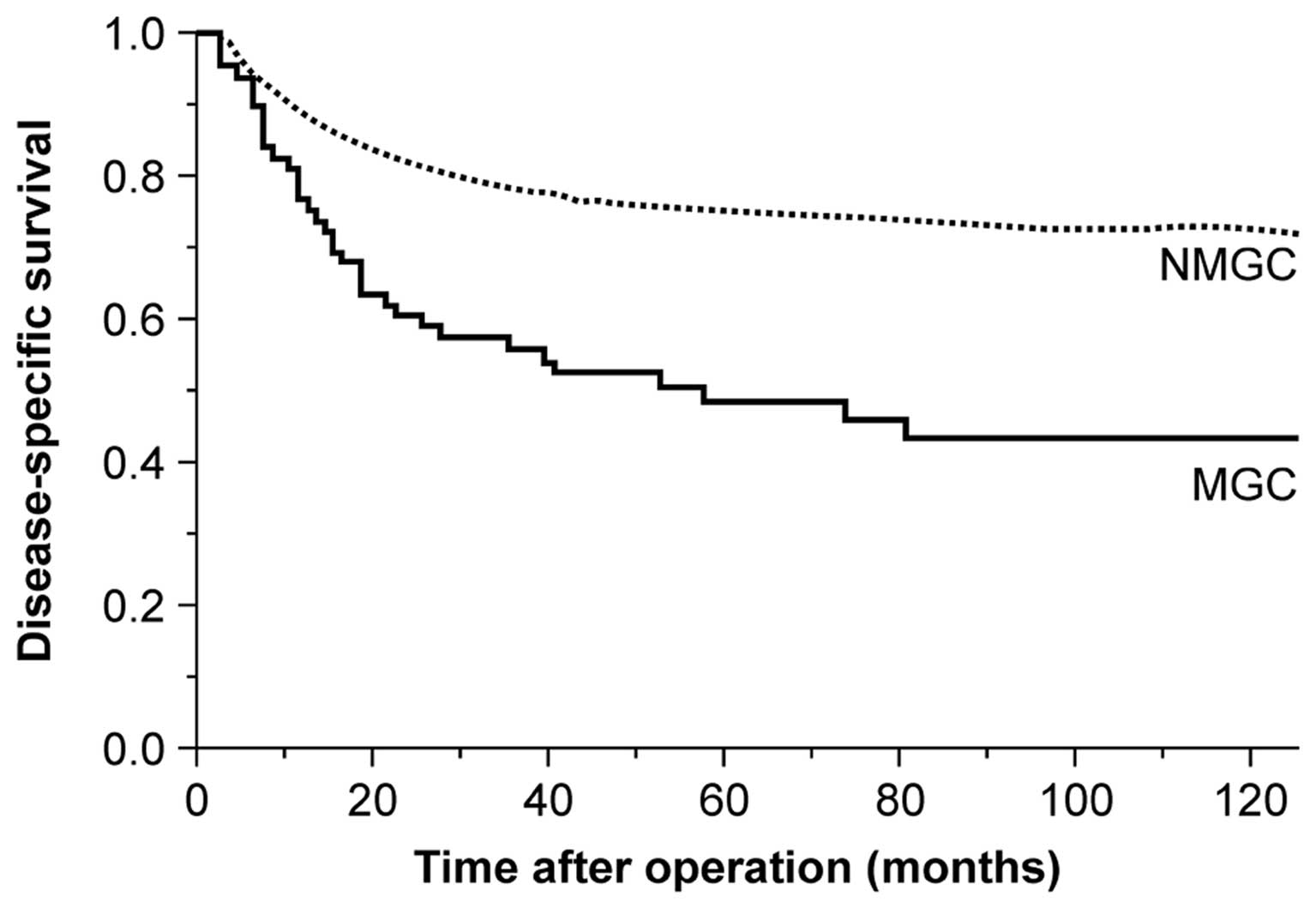
1–228 months). Fig. 1 shows the
postoperative disease-specific survival curves of all the patients.
The disease-specific survival rate of MGC patients was
significantly lower compared to that of NMGC patients (P<0.001).
The 5- and 10-year survival rates of MGC patients were 48.7 and
75.2%, respectively, whereas the corresponding rates for NMGC
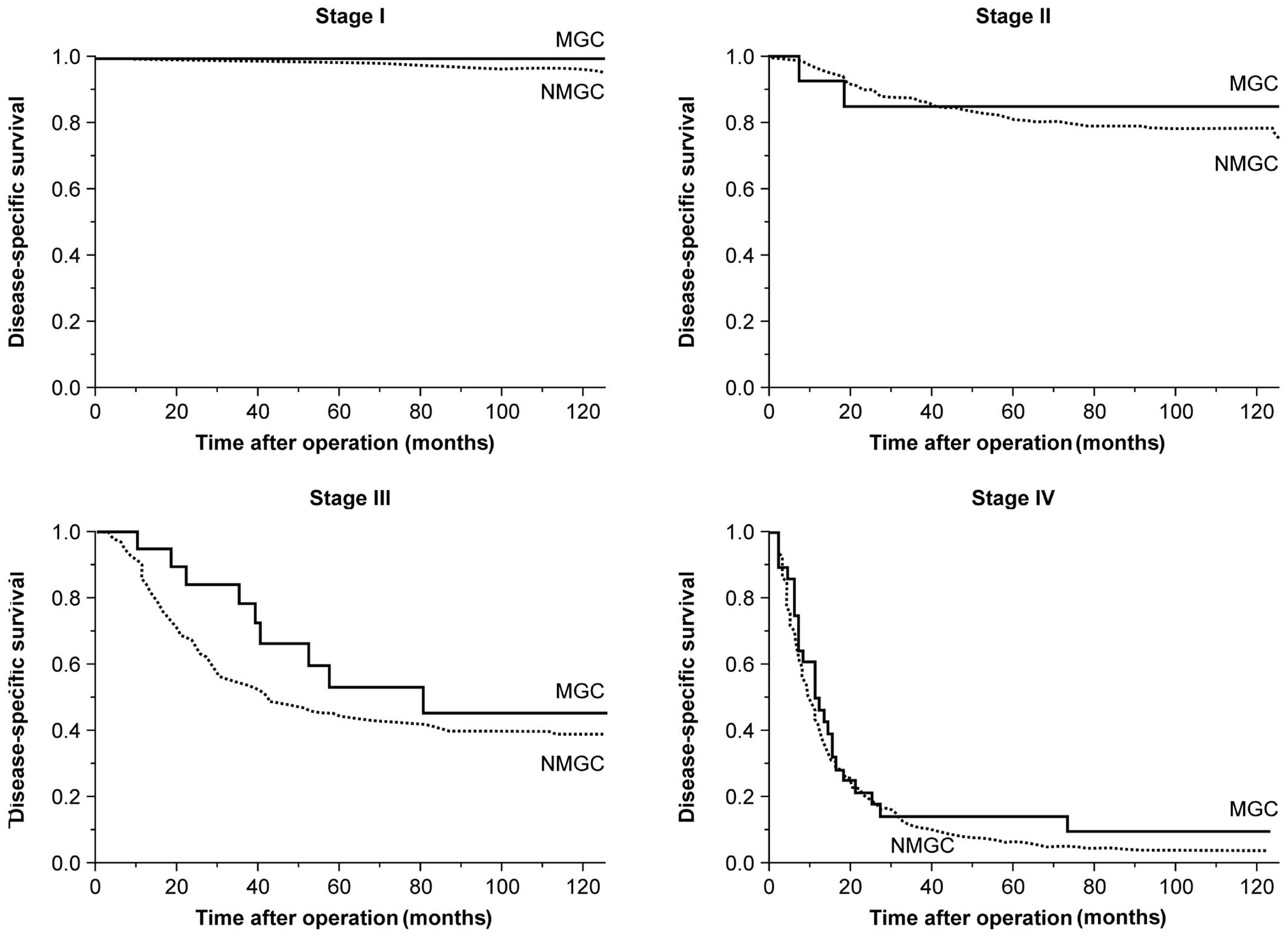
patients were 43.6 and 72.9%, respectively. However, when survival
was compared between MGC and NMGC patients according to disease
stage, no significant differences in 5- and 10-year survival rates
were observed between the two groups (Fig. 2 and Table III).
 | Table IIIComparison of 5- and 10-year survival
by disease stage between MGC and NMGC patients. |
Table III
Comparison of 5- and 10-year survival
by disease stage between MGC and NMGC patients.
| MGC (n=70) | NMGC (n=
2,492) | |
|---|
|
|
| |
|---|
| 5-year | 10-year | 5-year | 10-year | |
|---|
| Stage | (%) | (%) | (%) | (%) | P-value |
|---|
| I |
100.0 |
100.0 |
98.5 |
96.9 |
0.690 |
| II |
85.1 |
85.1 |
81.1 |
78.5 |
0.968 |
| III |
53.2 |
45.6 |
44.0 |
39.1 |
0.105 |
| IV |
14.3 |
9.5 |
6.4 |
4.1 |
0.386 |
Multivariate analysis of prognostic
factors
The univariate analysis revealed that tumor size,
macroscopic type, depth of invasion, lymph node metastasis,
lymphatic vessel invasion, venous invasion and peritoneal
metastasis were statistically predictive of 5-year disease-free
survival in MGC patients (Table
IV). Of these 7 factors, peritoneal metastasis was determined
as a relevant factor by the Cox proportional hazards model (odds
ratio, 3.00; P=0.011). When all the investigated gastric cancer
patients were analyzed, the Cox proportional hazards model revealed
that tumor size, macroscopic type, depth of invasion, lymph node
metastasis, peritoneal metastasis and hepatic metastasis were
significant predictive factors for survival. However, histological
type was not an independent prognostic factor (MGC vs. NMGC; odds
ratio, 1.41; P=0.062) (Table
V).
 | Table IVUnivariate and multivariate analyses
of prognostic factors for MGC patients. |
Table IV
Univariate and multivariate analyses
of prognostic factors for MGC patients.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| No. | 5-year
disease-free | | Odds | | |
|---|
| Factors | (n=70) | survival rate
(%) | P-value | ratio | 95% CI | P-value |
|---|
| Age (years) | | |
0.870 | | | |
|
≥65 | 47 |
45.2 | | | | |
|
<65 | 23 |
52.2 | | | | |
| Gender | | |
0.291 | | | |
|
Male | 50 |
50.7 | | | | |
|
Female | 20 |
43.8 | | | | |
| Tumor size
(mm) | | |
<0.001a |
1.15 | 0.48–2.98 |
0.755 |
|
≥80 | 35 |
28.4 | | | | |
|
<80 | 35 |
70.8 | | | | |
| Tumor location
(n=61) | | |
0.803 | | | |
|
Lower | 33 |
52.9 | | | | |
| Middle,
upper | 28 |
56.9 | | | | |
| Macroscopic type
(n=58) | | | 0.014a |
1.65 | 0.74–4.09 |
0.231 |
|
Borrmann 3, 4 | 35 |
30.4 | | | | |
|
Borrmann 1, 2 | 23 |
58.4 | | | | |
| Depth of
invasion | | |
<0.001a |
1.93 | 0.27–39.5 |
0.546 |
| T3,
T4 | 56 |
37.4 | | | | |
| T1,
T2 | 14 |
100.0 | | | | |
| Lymph node
metastasis | | |
<0.001a |
2.97 | 0.70–16.1 |
0.145 |
| N2,
N3 | 44 |
32.2 | | | | |
| N0,
N1 | 26 |
77.8 | | | | |
| Lymphatic
invasion | | | 0.008a |
1.46 | 0.28–6.04 |
0.629 |
| ly2,
ly3 | 54 |
39.7 | | | | |
| ly0,
ly1 | 16 |
79.1 | | | | |
| Venous
invasion | | | 0.004a |
1.05 | 0.24–3.62 |
0.948 |
| v1 | 51 |
39.8 | | | | |
| v0 | 19 |
74.8 | | | | |
| Peritoneal
metastasis | | |
<0.001a |
3.00 | 1.30–7.04 | 0.011a |
|
Positive | 17 |
11.8 | | | | |
|
Negative | 53 |
60.5 | | | | |
| Hepatic
metastasis | | | | | | |
|
Positive | 0 | - | | | | |
|
Negative | 70 |
48.7 | | | | |
 | Table VUnivariate and multivariate analyses
of prognostic factors for all gastric cancer patients. |
Table V
Univariate and multivariate analyses
of prognostic factors for all gastric cancer patients.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| No. | Disease-free | | Odds | | |
|---|
| Factors | (n=2,562) | survival rate
(%) | P-value | ratio | 95% CI | P-value |
|---|
| Age (years) | | | 0.038a |
1.10 | 0.92–1.30 |
0.278 |
|
≥65 | 1,443 |
72.6 | | | | |
|
<65 | 1,119 |
76.8 | | | | |
| Gender | | |
0.711 | | | |
|
Male | 1,753 |
50.7 | | | | |
|
Female | 809 |
43.8 | | | | |
| Tumor size
(mm) | | |
<0.001a |
1.31 | 1.09–1.57 | 0.004a |
|
≥80 | 576 |
34.2 | | | | |
|
<80 | 1,986 |
85.8 | | | | |
| Tumor location
(n=2,348) | | |
0.988 | | | |
|
Lower | 1,096 |
78.9 | | | | |
| Middle,
upper | 1,252 |
79.4 | | | | |
| Macroscopic type
(n=993) | | |
<0.001a |
1.47 | 1.20–1.80 |
<0.001a |
|
Borrmann 3, 4 | 624 |
30.7 | | | | |
|
Borrmann 1, 2 | 369 |
58.8 | | | | |
| Depth of
invasion | | |
<0.001a |
3.21 | 1.97–5.68 |
<0.001a |
| T3,
T4 | 984 |
38.3 | | | | |
| T1,
T2 | 1,578 |
97.1 | | | | |
| Lymph node
metastasis | | |
<0.001a |
2.52 | 2.01–3.18 |
<0.001a |
| N2,
N3 | 680 |
26.5 | | | | |
| N0,
N1 | 1,882 |
91.6 | | | | |
| Lymphatic
invasion | | |
<0.001a |
1.30 | 0.95–1.81 |
0.104 |
| ly2,
ly3 | 1,059 |
39.7 | | | | |
| ly0,
ly1 | 1,503 |
79.1 | | | | |
| Venous
invasion | | |
<0.001a |
1.03 | 0.84–1.25 |
0.798 |
| v2,
v3 | 293 |
29.2 | | | | |
| v0,
v1 | 2,269 |
80.2 | | | | |
| Peritoneal
metastasis | | |
<0.001a |
3.06 | 2.49–3.74 |
<0.001a |
|
Positive | 170 |
5.0 | | | | |
|
Negative | 2,392 |
79.4 | | | | |
| Hepatic
metastasis | | |
<0.001a |
3.45 | 2.56–4.59 |
<0.001a |
|
Positive | 75 |
56.5 | | | | |
|
Negative | 2,487 |
76.6 | | | | |
| Histopathological
type | | | |
1.41 | 0.98–2.10 |
0.062 |
| MGC | 70 |
48.7 |
<0.001a | | | |
| NMGC | 2,492 |
75.2 | | | | |
Discussion
Although gastric carcinoma is one of the most common
malignancies, its histological classification remains
controversial. The incidence of MGC reportedly varies between 2.6
and 6.6% (4–8). In our cohort of 2,562 gastric cancer
patients, 70 MGC and 2,492 NMGC cases were identified, with a 2.7%
incidence of MGC.
Although a number of previous survival studies have
attempted to compare carcinomas with and without mucinous
characteristics, MGC remains a histological subtype of unclear
prognosis. In this study, we investigated various
clinicopathological characteristics, including age, gender, tumor
location, tumor size, macroscopic type, lymphovascular invasion,
peritoneal metastasis, hepatic metastasis and tumor-node-metastasis
(TNM) stage. Kunisaki et al (4) and Hyung et al (8) reported no significant differences in
tumor size between MGC and NMGC patients. Furthermore, Zhang et
al (6) suggested that tumor
size, depth of invasion and lymph node metastasis were not
associated with MGC and NMGC. However, we observed that MGC and
NMGC differed in tumor size, macroscopic type, lymphovascular
invasion, peritoneal metastasis and TNM stage, which was in
agreement with the findings of Adachi et al (7) and Yin et al (14).
In this study, only 6 of 70 MGC patients were
diagnosed with early-stage disease. Our results also indicated that
the incidence of early-stage diseases was lower in MGC compared to
that in NMGC cases (8.6 vs. 55.1%). Several previous reports have
described the rarity of early-stage gastric cancer. Lim et
al (9) reported that the
incidence of early-stage MGC was only 6.5% compared to 26.0% in
NMGC cases, whereas those rates were 20.0 and 44.6%, respectively,
in a study by Kunisaki et al (4). Therefore, it is necessary to compare
the clinicopathological significance according to disease stage. We
also investigated the clinicopathological characteristics of stage
III and IV MGC and NMGC cases and found that the two groups did not
differ in tumor size, macroscopic type, lymphovascular invasion and
TNM stage. Additionally, peritoneal metastasis was more frequently
observed in MGC, whereas hepatic metastasis was more common in NMGC
cases. The rare incidence of hepatic metastasis in MGC was in
accordance with the results reported by Kawamura et al
(5).
The presence of a mucinous component is generally
associated with poor prognosis in colorectal cancer patients
(15). However, such a prognostic
correlation is less well defined in MGC. Several studies reported a
poor prognosis for MGC patients (5, 9,
10), while others suggested no
significant prognostic differences between MGC and NMGC (7, 11).
We observed that the 5-year survival rate of MGC patients was worse
compared to that of NMGC patients. However, no such significant
differences in survival rates were observed between the two groups
when the patients were stratified according to their disease stage.
Our results were in agreement with those of Yasuda et al
(11) and Kawamura et al
(5). Furthermore, the multivariate
analysis demonstrated that mucinous histological type was not a
prognostic indicator in patients with gastric cancer. Thus, our
findings suggested that the main factor affecting the poorer
prognosis of MGC compared to that of NMGC was the more frequent
incidence of advanced-stage disease at diagnosis, rather than the
aggressive biological behavior of MGC. However, the reason why MGC
is usually diagnosed at an advanced stage remains unclear. Previous
studies suggested the following possibilities: (i) MGC is
considered to initially arise as a typical adenocarcinoma, which
then becomes MGC as the tumor progresses and such a progression may
be considered as a dedifferentiation process; (ii) as a tumor
invades the gastric wall, the intraluminal excretion of mucin
decreases and an increasing deposition of mucin leads to the
intramural accumulation; and (iii) MGC is mainly located in the
submucosal or deeper layer, which may also be explained by the
intramural accumulation of mucin (7, 8,
14). However, the origin and
progression of MGC remain poorly understood.
In conclusion, our results indicated that MGC is
rare and mainly detected at an advanced stage, with a poorer
overall prognosis compared to that of NMGC. However, the prognosis
of MGC according to disease stage was similar to that of NMGC.
Therefore, the MGC histological subtype was not found to be an
independent prognostic factor of gastric cancer. Further
investigation on the origin and progression of MGC is required to
advance this field.
References
|
1
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer. 37
(Suppl 8):4–66. 2001.PubMed/NCBI
|
|
2
|
Santoro R, Carboni F, Lepiane P, Ettorre
GM and Santoro E: Clinicopathological features and prognosis of
gastric cancer in young European adults. Br J Surg. 94:737–742.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee HH, Song KY, Park CH and Jeon HM:
Undifferentiated-type gastric adenocarcinoma: prognostic impact of
three histological types. World J Surg Oncol.
10(254)2012.PubMed/NCBI
|
|
4
|
Kunisaki C, Akiyama H, Nomura M, Matsuda
G, Otsuka Y, Ono HA and Shimada H: Clinicopathologic
characteristics and surgical outcomes of mucinous gastric
carcinoma. Ann Surg Oncol. 13:836–842. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawamura H, Kondo Y, Osawa S, et al: A
clinicopathologic study of mucinous adenocarcinoma of the stomach.
Gastric Cancer. 4:83–86. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang M, Zhu GY, Zhang HF, Gao HY, Han XF
and Xue YW: Clinicopathologic characteristics and prognosis of
mucinous gastric carcinoma. J Surg Oncol. 102:64–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adachi Y, Mori M, Kido A, Shimono R,
Maehara Y and Sugimachi K: A clinicopathologic study of mucinous
gastric carcinoma. Cancer. 69:866–871. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hyung WJ, Noh SH, Shin DW, Yoo CH, Kim CB,
Min JS and Lee KS: Clinicopathologic characteristics of mucinous
gastric adenocarcinoma. Yonsei Med J. 40:99–106. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim SW, Kim DY, Kim YJ and Kim SK:
Clinicopathologic features of mucinous gastric carcinoma. Dig Surg.
19:286–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu CY, Yeh HZ, Shih RT and Chen GH: A
clinicopathologic study of mucinous gastric carcinoma including
multivariate analysis. Cancer. 83:1312–1318. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasuda K, Shiraishi N, Inomata M,
Shiroshita H, Ishikawa K and Kitano S: Clinicopathologic
characteristics of early-stage mucinous gastric carcinoma. J Clin
Gastroenterol. 38:507–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watanabe H, Jass JR and Sobin LH:
Histological typing of oesophageal and gastric tumors: WHO
international histological classification of tumors. (2nd). Cancer.
66:2162–2167. 1990.
|
|
13
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin C, Li D, Sun Z, Zhang T, Xu Y, Wang Z
and Xu H: Clinicopathologic features and prognosis analysis of
mucinous gastric carcinoma. Med Oncol. 29:864–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sadahiro S, Ohmura T, Saito T and Akatsuka
S: An assessment of the mucous component in carcinoma of the colon
and rectum. Cancer. 64:1113–1116. 1989. View Article : Google Scholar : PubMed/NCBI
|