Introduction
Lung cancer is the leading cause of cancer-related
mortality. Non-small-cell lung cancer (NSCLC) represents the
majority of lung cancer cases. Lobectomy is the standard therapy
used for stage I peripheral NSCLC. However, lobectomy is a major
surgical procedure that is often associated with a clinically
significant decline in pulmonary function. Furthermore, due to the
usually high rate of comorbidities and poor baseline pulmonary
function, patients with early NSCLC are often considered medically
inoperable. Historically, the treatment outcomes of inoperable
patients with clinical stage I NSCLC have been poor (1). A large field must be irradiated to
account for respiratory tumour motion; as a consequence,
conventional or standard radiotherapy for early-stage NSCLC
patients usually leads to excessive exposure of normal lung tissue.
Three-dimensional conformal radiotherapy (3DCRT) has been widely
applied for the treatment of lung malignancies. The lower
conformality of 3DCRT results in a larger volume receiving the
therapeutic dose, which may be preferable for a moving target. In
addition, locoregional control and survival are typically
disappointing with conventional radiation therapy. Clinically
significant radiation pneumonitis usually develops in 13–37% of
patients receiving radical dose radiotherapy against lung cancer
(2).
Over the last few years, stereotactic body radiation
therapy (SBRT) has been applied as a standard treatment option in
the treatment against inoperable early-stage peripheral NSCLC.
CyberKnife (CK) is a non-invasive robotic system, which allows
delivery of SBRT through precise, nearly real-time image-guided
tracking of a mobile target. Previous studies (3–5)
demonstrated excellent local control rates of 90–97% at 2–3 years'
follow-up, with minimal associated toxicities.
There are currently no randomized trials comparing
the efficacy and acute toxicity of CK and 3DCRT in thoracic
malignancies. The most common acute toxicity is radiation
pneumonitis following lung radiotherapy. Our objective was to
compare the efficacy and acute toxicity of the se two techniques
against inoperable stage I peripheral NSCLC.
Materials and methods
Patient eligibility
We retrospectively investigated a total of 68
patients with pathologically confirmed clinical stage I peripheral
NSCLC between 2012 and 2013. A total of 38 patients received 3DCRT
with flattened beams, whereas the remaining 30 patients were treated
with CK. The main characteristics of the patient cohorts are
summarized in Table I. Thoracic
computed tomography (CT) imaging and routine pulmonary function
tests (PFTs) were performed prior to treatment. The patients were
considered ineligible for surgical resection due to advanced age or
the presence of comorbidities.
 | Table I.Patient baseline characteristics. |
Table I.
Patient baseline characteristics.
| | Treatment groups | |
|---|
| |
| |
|---|
| Variables | Entire cohart
(n=68) | 3DCRT (n=38) | CK (n=30) | P-value |
|---|
| Age (years) | | | |
0.919 |
|
<70 | 29 | 16 | 13 | |
| ≥70 | 39 | 22 | 17 |
| Gender | | | |
0.243 |
| Male | 40 | 20 | 20 | |
|
Female | 28 | 18 | 10 | |
| ECOG PS | | | |
0.340 |
| 0-1 | 41 | 21 | 20 | |
| 2 | 27 | 17 | 10 | |
| Smoking | | | |
0.174 |
| Yes | 38 | 24 | 14 |
| No | 30 | 14 | 16 |
| Pre-FEV1/FVC | | | |
0.660 |
|
<68% | 23 | 12 | 11 | |
| ≥68% | 45 | 26 | 19 |
| Tumor location | | | |
0.697 |
|
Upper | 47 | 27 | 20 | |
|
Lower | 21 | 11 | 10 | |
| Clinical stage | | | |
0.382 |
|
T1N0M0 | 24 | 12 | 12 | |
|
T2aN0M0 | 44 | 26 | 18 |
| Pathology of
NSCLC | | | |
0.265 |
|
Squamous | 20 | 11 | 9 | |
|
Adenocarcinoma | 40 | 20 | 20 | |
|
Other | 8 | 7 | 1 | |
Fiducial placement
All the stage I NSCLC patients were implanted using
fiducial markers with the Synchrony ® Respiratory Motion
Tracking system (6). Under
conscious sedation and local anesthesia, 3–5 gold fiducials
measuring 0.8 mm in diameter by 3 mm in length (Civco Medical
Solutions, Orange City, IA, USA) were placed with adequate spacing
(1–2 cm) in or near tumours under CT guidance (7, 8).
Treatment planning
We treated a group of patients with the CyberKnife
frameless robotic radiosurgery system (Accuray, Inc., Sunnyvale,
CA, USA). We obtained fine-cut (1.5-mm) treatment planning CTs 7–10
days following fiducial placement during a full-inhalation
breath-hold. Gross tumour volume (GTV) was contoured with lung
windows. The GTV margin was expanded by 5 mm to set the planning
treatment volume (PTV). All the critical thoracic structures and
the lungs were contoured to ensure that incidental radiation
delivered to these structures was limited according to the reports
of the American Association of Physicists in Medicine Task Group
101. A treatment plan from Multiplan software (Accuray, Inc.) was
made using the CyberKnife non-isocentric, inverse-planning
ray-tracing algorithm with tissue density heterogeneity corrections
for lung. Lower doses within the range of 42–60 Gy in three
fractions were prescribed when concerns regarding adjacent critical
structures arose and when patients were considered to exhibit
severe pulmonary dysfunction. The biologically effective dose (BED)
was 100.8-180 Gy for patients undergoing CK treatment. The
radiation dose was prescribed to an isodose line that covered ≥ 95%
of the PTV and caused the 30-Gy isodose contour to extend a minimum
of 1 cm from the GTV. The percentage of the total lung volume
receiving ≥ 15 Gy (V15) was limited to 15%.
Radiation was delivered with photon beams of 6 MV
from a linear accelerator (Elekta Precise; Elekta Oncology Systems,
Crawley, UK) in the 3DCRT group. Each of the patients was
irradiated for 60 Gy, 2 Gy/fraction, once per day, 5 days per week.
The BED was 72 Gy for the patients receiving 3DCRT treatment.
Radiation Therapy Planning software (Pinnacle3; Philips Medical
Systems, Fitchburg, WI, USA) was used to design the radiation plan.
In the 3DCRT plans, due to the unavailability of 4DCT imaging,
larger margins were used to define the PTV (10, 10 and 15 mm in the
latero-lateral, antero-posterior and cranio-caudal directions,
respectively) to account for respiratory motion. The lungs, heart
and spinal cord were considered as organs at risk (OARs). The
planning objective was to cover 95% of the volume with 95% of the
dose for the PTV. The constraints for the OARs were Dmax <20 Gy
for the spinal cord and Dmax <30 Gy for the heart. For the joint
lungs, exclusive of PTV, the following constraints were set: V30Gy
<20% and a mean lung dose <4 Gy.
The BED was calculated with the following linear
quadratic formula: BED = (nd) [1+d/(α/β)]. Factor α/βwas assumed to
be 10 Gy, with the variables n and d representing the number of
fractions and the dose per fraction, respectively.
Treatment delivery
In brief, pretreatment fluoroscopy confirmed that
fiducial motion was associated with tumour motion. The patients
were then transferred to the CK suite and laid supine on the
treatment table with their arms at their sides. Three red
light-emitting diodes (LEDs) were placed above the patient's
anterior torso directing toward the camera array. Fiducials were
located with orthogonal X-ray imagers. A correlation model was
formed between the LEDs tracked continuously by the camera array
and the fiducial positions imaged periodically by the X-ray
targeting system. During treatment delivery the tumour position was
tracked through the live camera array signal and correlation model;
the robotic arm moved the linear accelerator to maintain precise
alignment with the tumour throughout the respiratory cycle.
Fiducials were imaged prior to delivery of every third beam to
check targeting accuracy and to update the correlation model.
The patients in the 3DCRT group were placed in a
supine position with their hands on their head and fingers
interlocked. The body position was fixed and respiratory movement
was appropriately limited with a vacuum pad. By planning images
transmitted by the network from the three-dimensional treatment
planning system, the patients received conventional external
radiotherapy.
Follow-up studies
The patients were followed up for 1 year after the
date of CK or 3DCRT treatment completion. Clinical outcome was
evaluated through physical examination and thoracic CT imaging
prior to and after treatment. Toxicities were graded according to
the Radiation Therapy Oncology Group criteria (9, 10).
Clinical outcome was tumour control defined as disappearance,
shrinkage or growth of the tumour, assessed according to the
Response Evaluation Criteria in Solid Tumours, which are defined as
follows: complete remission (CR), disappearance of all target
lesions; partial remission, 30% decrease in the sum of the longest
diameter of target lesions; progressive disease, 20% increase in
the sum of the longest diameter of target lesions; stable disease,
small changes that do not meet the abovementioned criteria
(11). To asses the effects of
radiotherapy on functional tests, routine PFTs were performed at 1,
3, 6 and 12 months, including forced vital capacity (FVC), forced
expiratory volume during the first second (FEV1) and carbon
monoxide diffusion capacity (DCLO).
Statistical analysis
The correlations between FEV1/FVC prior to treatment
(pre-FEV1/FVC) and radiation pneumonitis were evaluated by using
Spearman's rank correlation. A receiver operating characteristic
(ROC) curve was generated to assess the pre-FEV1/FVC for radiation
pneumonitis. The pre-FEV1/FVC values were divided into two groups
using the threshold of pre-FEV1/FVC selected by the ROC curve. The
differences in categorical variables between the CK and 3DCRT
groups were compared using Chi-square tests. Univariate and
multivariate logistic regression analyses were used to indicate the
association between radiation pneumonitis and a number of
variables, including age, gender, Eastern Cooperative Oncology
Group performance status, radiation therapy techniques and
pre-FEV1/FVC. The comparison of radiation pneumonitis between the
two subgroups (pre-FEV1/FVC <68% vs. ≥68%) with different
radiation therapy techniques was assessed using the rank-sum
test.
The differences between pre- and post-treatment in
terms of PFTs for the two groups were assessed using the t-test for
statistics. All the analyses were performed using the SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistical ly significant difference.
Results
Patients
A total of 68 consecutive patients diagnosed with
NSCLC were treated with definitive radiation between 2012 and 2013,
with 38 patients receiving 3DCRT and 30 receiving CK. Among the
patients treated with 3DCRT, 20 (53%) were men and 18 (47%) women,
with a median age of 75.7 years (range, 65–82 years). Among the
patients treated with CK, 20 (67%) were men and 10 (33%) women,
with a median age of 74.7 years (range, 66–83 years). There was no
statistically significant difference between the two treatment
groups regarding patient characteristics (Table I).
Correlation of different variables
with radiation pneumonitis
A positive correlation was found between
pre-FEV1/FVC and radiation pneumonitis (r=0.844, P=0.000). We
divided the 68 patients into two subgroups using the threshold of
pre-FEV1/FVC selected by the ROC curve. The binary univariate
logistic regression analysis revealed a significant association of
radiation pneumonitis with treatment method (3DCRT vs. CK) and
pre-FEV1/FVC (OR=12.651 and 21.793, respectively) (Table II). The analysis of these factors
by the multivariate logistic regression method demonstrated that
treatment method for grades 1 and 2 (OR = 7.866 and 11.334,
respectively) and pre-FEV1/FVC for grades 1, 2 and 3 (OR = 5.062,
11.498 and 15.042, respectively) were significant factors affecting
the risk of radiation pneumonitis P<0.05) (Table III), with an increased risk for
pre-FEV1/FVC <68% compared to pre-FEV1/FVC ≥68% and in the 3DCRT
compared to the CK group. There were 3 patients in the 3DCRT group
with grade 3 radiation pneumonitis, whereas there were none in the
CK group (the OR was infinite).
 | Table II.Result s of univariate logistic
regression analysis for the correlation with radiation
pneumonitis. |
Table II.
Result s of univariate logistic
regression analysis for the correlation with radiation
pneumonitis.
| Variables | B | SE | Wald | P-value | OR(95%CI) |
|---|
| Age (<70/≥70
years) |
-0.604 |
0.775 |
0.068 |
0.436 | 0.547
(0.120-2.499) |
| Gender
(male/female) |
0.423 |
1.830 |
0.053 |
0.817 | 1.526
(0.042-55.069) |
| ECOG PS | | | | | |
| 1/0 |
1.204 |
0.619 |
3.781 |
0.052 |
3.333(0.991-11.271) |
|
2/0 |
0.223 |
0.626 |
0.127 |
0.721 | 1.250
(0.367-4.262) |
| Smoking
(no/yes) |
-0.483 |
0.837 |
0.333 |
0.564 | 0.617
(0.120-3.182) |
| Pathology | | | | | |
|
Squamous/other |
0.499 |
1.241 |
0.162 |
0.688 | 1.647
(0.145-18.753) |
|
Adenocarcinoma/other |
-0.027 |
1.906 |
0.000 |
0.989 | 0.973
(0.023-40.758) |
| Tumor location
(upper/lower) |
-0.149 |
0.723 |
0.042 |
0.837 | 0.862
(0.209-3.554) |
| Clinical stage
(T1/T2a) |
-0.845 |
0.725 |
1.358 |
0.244 |
0.430(0.104-1.778) |
| Treatment method
(3DCRT/CK) |
2.538 |
0.797 |
10.148 |
0.001a | 12.651
(2.655-60.285) |
| Pre-FEV1/FVC
(<68%/≥68%) |
3.082 |
0.913 |
11.398 |
0.001a | 21.793
(3.642-130.400) |
| Constant |
-1.825 |
2.313 |
0.623 |
0.430 | 0.161 |
 | Table III.Result s of multivariate logistic
regression analysis for the correlation with radiation
pneumonitis. |
Table III.
Result s of multivariate logistic
regression analysis for the correlation with radiation
pneumonitis.
| Radiation
pneumonitis | B | STH | Wald | P-value | OR (95% CI) |
|---|
| Grade 1 | | | | | |
|
Intercept |
-2.291 |
0.660 |
12.052 |
0.001 | |
|
Pre-FEV1/FVC
(<68%/≥68%) |
1.622 |
0.729 |
4.955 |
0.026 | 5.062
(1.214-21.113) |
|
3DCRT/CK |
2.063 |
0.717 |
8.276 |
0.004 | 7.866
(1.930-32.066) |
| Grade 2 | | | | | |
|
Intercept |
-3.516 |
0.955 |
13.566 |
0.000 | |
|
Pre-FEV1/FVC
(<68%/≥68%) |
2.442 |
0.853 |
8.206 |
0.004 | 11.498
(2.163-61.134) |
|
3DCRT/CK |
2.428 |
0.940 |
6.673 |
0.010 | 11.334
(1.796-71.517) |
| Grade 3 | | | | | |
|
Intercept |
-21.846 |
0.839 |
678.731 |
0.000 | |
|
Pre-FEV1/FVC
(<68%/≥68%) |
2.711 |
1.399 |
3.752 |
0.050 | 15.042
(0.969-233.605) |
|
3DCRT/CKa | | | | | |
Comparison of radiation pneumonitis
grades between 3DCRT and CK in the pre-FEV1/FVC <68% and ≥68%
subgroups
In pre-FEV1/FVC <68% and ≥68% groups, the grades
of radiation pneumonitis with CK treatment were lower compared to
those with 3DCRT treatment (mean rank, 8.68 vs. 15.04 and 18.08 vs.
27.67, respectively). There were significant differences between
the 3DCRT and CK treatment in the pre-FEV1/FVC <68% and ≥68%
subgroups (P=0.023 and 0.002) (Table
IV).
 | Table IV.Comparison of different grades of
radiation pneumonitis between 3DCRT and CK treatment in two
subgroups (pre-FEV1/FVC <68% and ≥68%). |
Table IV.
Comparison of different grades of
radiation pneumonitis between 3DCRT and CK treatment in two
subgroups (pre-FEV1/FVC <68% and ≥68%).
| Pre-FEV1/FVC
(<68%) | Pre-FEV1/FVC
(≥68%) |
|---|
|
|
|
|---|
| Grade | 3DCRT | CK | 3DCRT | CK |
|---|
| 0 | 1 | 5 | 14 | 19 |
| 1 | 3 | 3 | 9 | 1 |
| 2 | 5 | 3 | 3 | 0 |
| 3 | 3 | 0 | 0 | 0 |
| Mean rank | 15.04 | 8.68 | 27.67 | 18.08 |
| P-value | 0.023 | 0.002 | | |
Comparison of pulmonary function tests
between 3DCRT and CK
Considering the effects of radiotherapy on
functional tests, there were no significant differences in
parameters such as FVC and FEV1 at pre-and post-treatment (1, 3, 6
and 12 month s) in the 3DCRT group. However, there were
statistically significant decreases in the mean DLCO in the 3DCRT
group at 6 and 12 months post-treatment. Additionally, there was a
distinct decrease in the mean DLCO in the two subgroups
(pre-FEV1/FVC <68% and ≥68%) of the 3DCRT group at 6 and 12
months. In the CK group, there was no significant change in FVC,
FEV1 or DCLO (Fig. 1).
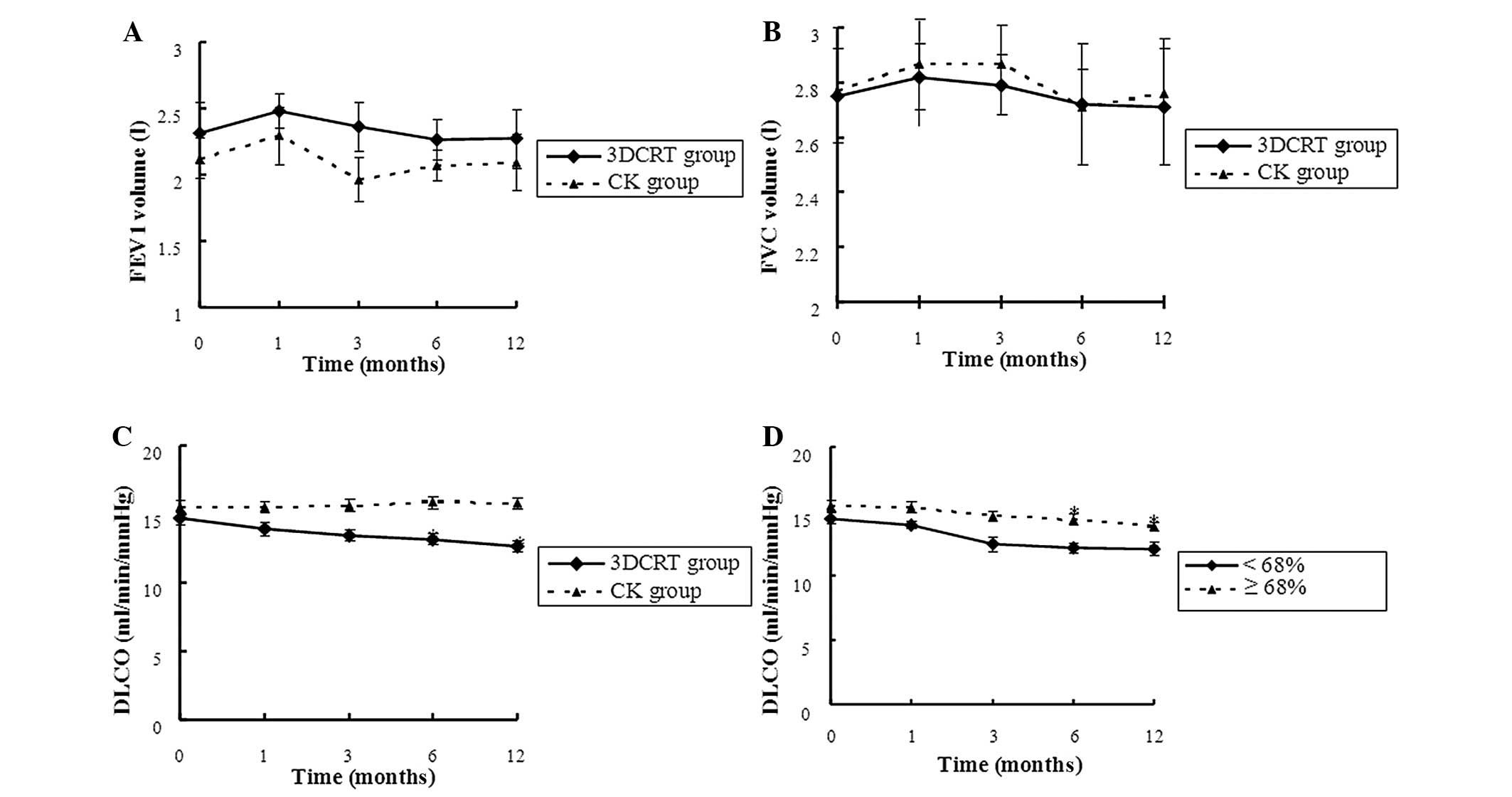 | Figure 1.Comparison of pulmonary function tests
following 3DCRT and CK treatment. Differences in (A) FEV1, (B) FVC
and (C) DLCO pre- and post- treatment (1, 3, 6 and 12 months) in
the 3DCRT and CK groups. (D) Differences in DLCO at pre- and post-
treatment (1, 3, 6 and 12 months) in the two subgroups
(pre-FEV1/FVC <68 and ≥68%) of the 3DCRT group.* The follow-up
results were compared to the values prior to treatment (P<0.05).
3DCRT, three-dimensional conformal radiotherapy; CK, CyberKnife;
FEV1, forced expiratory volume during the first second; FVC, forced
vital capacity; DLCO, carbon monoxide diffusion capacity. |
All the patients underwent follow-up evaluation at
3, 6 and 12 months. No patients were lost to follow-up and no
patient succumbed to the disease within that time period. The local
control rate at 1 year was 100% in patients treated with CK and
97.4% in those treated with 3DCRT. The follow-up results at 6
months were compared to those at 3 months; and the follow-up
results at 12 months were compared to those at 6 months.
Response to treatment on CT scan
As shown in Table
V, the major radiological response (CR), as evaluated by CT
scan at 3, 6 and 12 months, was recorded in 9/38 (23.7%) vs. 10/38
(33.3%), 13/38 (34.2%) vs. 12/30 (40.0%) and 13/38 (34.2%) vs.
12/30 (40.0%) of patients treated with 3DCRT vs. CK,
respectively.
 | Table V.Response to treatment on computed
tomography morphological scan. |
Table V.
Response to treatment on computed
tomography morphological scan.
| 3DCRT, no. (%) | CK, no. (%) |
|---|
|
|
|
|---|
| Response | 3 months | 6 months | 12 months | 3 months | 6 months | 12 months |
|---|
| Complete
remission | 9 (23.7%) | 13 (34.2%) | 13 (34.2%) | 10 (33.3%) | 12 (40.0%) | 12 (40.0%) |
| Partial
remission | 15 (39.5%) | 17 (44.7%) | 14 (36.8%) | 12 (40.0%) | 13 (43.3%) | 7 (23.3%) |
| Stable disease | 13 (34.2%) | 8 (21.1%) | 11 (29.0%) | 8 (26.7%) | 5 (16.7%) | 11 (36.7%) |
| Progressive
disease | 1
(2.6%)a | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Discussion
CK is a new type of SBRT, which is able to deliver
radiation from more angles utilizing image guidance and real-time
target tracking. CK treatment has been shown to be very effective
for stage I NSCLC in several studies published over the last few
years (3, 4, 6,
12, 13).
Numerous studies demonstrated that an increase in
radiological doses may increase the tumour control rates (14). However, further dose escalation
with broad-field radiotherapy may induce higher toxicity, as
healthy tissues also receive a higher dose of radiation, which
decreases the patients' survival rates (15).
The binary univariate and multivariate logistic
regression analys es demonstrated that the risk of radiation
pneumonitis is associated with treatment methods and pre-FEV1/FVC.
Patients with underlying lung diseases may exhibit poor lung
function, lower-than-normal FVC and FEV1, insufficient respiratory
ventilation and air exchange function and low lung compliance;
thus, such patients are more likely susceptible to radiation
pneumonitis, as reported by a number of experimental studies
(15–17). In all the patients, the risk and
grade of radiation pneumonitis were higher in the 3DCRT compared to
those in the CK group. In the ROC curve of the pre-FEV1/FVC of
patients who were divided into two subgroups, the threshold was set
at FEV1/FVC=68%. In the two groups, the incidence of radiation
pneumonitis in the 3DCRT group was significantly higher compared to
that in the CK group. Several studies have established an
association between the risk of radiation pneumonitis and
dose-volume distribution in the lung (18). CK appeared to be an appropriate
option for further increasing the tumour biological equivalent
dose, without increasing normal tissue toxicity.
In our study, we compared these two radiotherapy
approaches according to lung function changes prior to and
following radiotherapy. When all the patients were evaluated at 1,
3, 6 and 12 months, no significant changes in FVC and FEV1 were
observed, which was possibly due to offsetting between
radiation-induced lung tissue damage and tumour regression-induced
ventilation function improvement. In the 3DCRT group, DLCO (a
measure of diffusion capacity) was significantly decreased; such a
tendency was not observed in the CK group. A previous study by
Lopez et al (19) indicated
that the percentage of the decrease in DLCO is closely associated
with severe radiation pneumonitis.
In lung cancer radiotherapy, pulmonary surfactant is
released into the alveolar cavity, leading to changes in alveolar
tension, alveolar collapse and atelectasis. Furthermore, the injury
of type II alveolar epithelial cells and hyperplasia of the
collagen fibers lead to alveolar wall and capillary endothelial
cell thickening. In addition, edematous fluid with in the cell
layer leads to the increased diffusion distance and impaired
diffusion.
CK successfully preserved the lung diffusion
capacity, as measured by PFTs. Our 3DCRT treatment group exhibited
a higher risk of radiation pneumonitis compared to that in the CK
group, which may be associated with the lower radiation doses to
normal tissues in the CK group and the reduced effect of these low
doses on diffusion function.
We evaluated the therapeutic effect following
radiotherapy through CT scan at different time points. The local
tumour control rate in the 3DCRT group reached 97.4%, while that of
the CK group reached 100%. The CR rates in the CK group at 3, 6 and
12 months were higher compared to those in the 3DCRT group, as
higher doses for tumour target volumes led to higher local tumour
control rates. The commonly accepted dose scheme of 60 Gy in three
fractions for peripheral lung tumours, which is equivalent to 150
Gy in 2 Gy fractions if α/βis 10 Gy (normalised total dose), was
adopted as our standard. When compared to 3DCRT, the average
exposure dose increased by 140%, significantly boosting the
biological doses, but with small exposure scopes for normal tissues
(20).
The mechanism accounting for higher biological doses
via CK and its low exposure dose targeting normal tissues is as
follows: the delivery of hundreds of radiation beams while
continuously tracking and compensating for respiratory tumour
motion ensures that the gross tumour and radial microscopic
extension are effectively treated. The higher the number of
spread-out non-coplanar beams, the more likely the system is to
form a rapid dose fall-off (steep dose gradient). The device used
in this study addresses the challenge of respiratory motion using
an image-guided real-time targeting/tracking system, resulting in a
significantly reduced targeting uncertainty. In addition, a
protracted fractionation schedule, which is often used to minimise
the risk of radiotherapy-related pneumonitis, may result in poorer
outcomes due to accelerated tumour cell repopulation during the
radiation course (21, 22). Precise radiotherapy by using CK
uses high single doses and few segmentation numbers, together with
several advantages that are absent in conventional radiotherapy.
For example, i) CK treatment does not require any method to limit
breathing due to tumour tracking; and ii) CK requires shorter
hospitalization time and is associated with tolerable adverse
reactions, thus it is easier to obtain the patients' consent. In
our study, CK was proven to be feasible and safe, while achie ving
excellent rates of local disease control with limited toxicity to
surrounding tissues and, in several cases, may be curative for
patients who are not candidates for surgery. In experiments
performed by other research centers, CK achieved higher
locoregional control and survival rates similar to wedge resection
(23).
CK has shown favorable prospects in stereotactic
radiosurgery. However, several problems regarding CK require
further investigation: i) although the administered exposure doses
and segmentation methods are currently based on biological
equivalent doses, the use of CK is, to a great extent,
experience-oriented and, in several recent literature reports,
long-term follow-up results supporting the effects of these dose
modes on partial tumour recurrence and data on future complications
for large-sample cases are not available; and ii) a significant
number of extracranial tumours require implanting metal particle
markers around the tumours, which may be associated with certain
complications.
Acknowledgements
This study was supported by a grant from the China
Postdoctoral Science Foundation (no. 20080431411).
References
|
1
|
Qiao X, Tullgren O, Lax I, et al: The role
of radiotherapy in treatment of stage I non-small cell lung cancer.
Lung Cancer. 41:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodrigues G, Lock M, D'Souza D, et al:
Prediction of radiation pneumonitis by dose-volume histogram
parameters in lung cancer - a systematic review. Radiother Oncol.
71:127–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown WT, Wu X, Fayad F, et al: Cyber
Knife radiosurgery for stage I lung cancer: results at 36 months.
Clin Lung Cancer. 8:488–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Voort van Zyp NC, Prévost JB,
Hoogeman MS, et al: Stereotactic radiotherapy with real-time tumor
tracking for non-small cell lung cancer: clinical outcome.
Radiother Oncol. 91:296–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Timmerman R, Paulus R, Galvin J, et al:
Stereotactic body radiation therapy for inoperable early stage lung
cancer. JAMA. 303:1070–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Collins BT, Vahdat S, Erickson K, et al:
Radical cyberknife radiosurgery with tumor tracking: an effective
treatment for inoperable small peripheral stage I non-small cell
lung cancer. J Hematol Oncol. 2:12009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banovac F, McRae D, Dieterich S, et al:
Percutaneous placement of fiducial markers for thoracic
malignancies. Robotic Radiosurgery: Treating Tumors that Move with
Respiration. Urschel HC, Kresel JJ, Luketich JD, Papiez L and
Timmerman RD: Springer-Verlag; Berlin: pp. 15–29. 2007
|
|
8
|
Yousefi S, Collins BT, Reichner CA, et al:
Complications of thoracic computed tomography-guided fiducial
placement for the purpose of stereotactic body radiation therapy.
Clin Lung Cancer. 8:252–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radiation Therary Oncology Group, . Acute
radiation morbidity scoring criteria. http://www.rtog.org/ResearchAssociates/AdverseEventReporting/AcuteRadiationMorbidityScoringCriteria.aspxAccessed.
May 1–2012
|
|
10
|
Radiation Therapy Oncology Group/European
Organization for Research Treatment of Cancer, . Late radiation
morbidity scoring schema. Available at. http://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiationMorbidityScoringSchema.aspxAccessed.
May 1–2012.
|
|
11
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vahdat S, Oermann EK, Collins SP, et al:
Cyber Knife radiosurgery for inoperable stage IA non-small cell
lung cancer:18F-fluorodeoxyglucose positron emission
tomography/computed tomography serial tumor response assessment. J
Hematol Oncol. 3:62010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collins BT, Erickson K, Reichner CA, et
al: Radical stereotactic radiosurgery with real-time tumor motion
tracking in the treatment of small peripheral lung tumors. Radiat
Oncol. 2:392007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehta M, Scrimger R, Mackie R, et al: A
new approach to dose escalation in non-small-cell lung cancer. Int
J Radiat Oncol Biol Phys. 49:23–33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robnett TJ, Machtay M, Vines EF, et al:
Factors predicting severe radiation pneumonitis in patients
receiving definitive chemoradiation for lung cancer. Int J Radiat
Oncol Biol Phys. 48:89–94. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kocak Z, Borst GR, Zeng J, et al:
Prospective assessment of dosimetric/physiologic-based models for
predicting radiation pneumonitis. Int J Radiat Oncol Biol Phys.
67:178–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lind PA, Marks LB, Hollis D, et al:
Receiver operating characteristic curves to assess predictors of
radiation-induced symptomatic lung injury. Int J Radiat Oncol Biol
Phys. 54:340–347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marks LB, Bentzen SM, Deasy JO, et al:
Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol
Phys. 76 (Suppl 3):S70–S76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lopez Guerra JL, Gomez D, Zhuang Y, et al:
Change in diffusing capacity after radiation as an objective
measure for grading radiation pneumonitis in patients treated for
non-small-cell lung cancer. Int J Radiat Oncol Biol Phys.
83:1573–1579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prevost JB, Voet P, Hoogeman M, et al:
Four-dimensional stereotactic radiotherapy for early stage
non-small cell lung cancer: a comparative planning study. Technol
Cancer Res Treat. 7:27–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gouders D, Maingon P, Paesmans M, et al:
Exclusive radiotherapy for non-small cell lung cancer. A
retrospective multicentric study. Rep Pract Oncol Radiother (Polish
Soc Rad Oncol). 8:7–14. 2003. View Article : Google Scholar
|
|
22
|
Sibley GS, Jamieson TA, Marks LB, et al:
Radiotherapy alone for medically inoperable stage I non-small-cell
lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys.
40:149–154. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen VJ, Oermann E, Vahdat S, et al: Cyber
Knife with tumor tracking: an effective treatment for high-risk
surgical patients with stage I non-small cell lung cancer. Front
Oncol. 2:92012. View Article : Google Scholar : PubMed/NCBI
|















