Introduction
According to the cancer stem cell model, the small
pool of self-renewing cancer stem cells must be eliminated in order
to eradicate the tumor (1–4). Acute myeloid leukemia (AML) is a
developmental disease characterized by clonal growth and subsequent
accumulation of myeloid blasts in the bone marrow (BM), which is
initiated and maintained by a subset of self-renewing leukemia stem
cells (LSCs). Thus far, human AML stem cells are the most
extensively characterized cancer stem cell population. LSCs share
numerous properties with hematopoietic stem cells (HSC) with the
ability of self-renewal. Thus, it has been suggested that the
clonal progression of preleukemia may occur in a succession of HSC
subclones until augmented or poorly regulated self-renewal pathways
are activated, leading to the emergence of final stage LSCs usually
at the level of a downstream progenitor (5).
A number of studies have shown that LSC-enriched
populations are resistant to various chemotherapy agents and are
therefore possibly responsible for the outgrowth of minimal
residual disease, which in turn is believed to cause relapse
(6,7).
Thus, the expression profile of LSCs specific cell surface markers
may be used as a prognosis factor to predict the drug response in
AML patients. Similar to the normal HSCs, AML-LSC are enriched in
the CD34+CD38− population (8). However, AML-LSCs also express certain
unique cell surface marker combinations, such as
CD123high CD117+, CD90+,
CD47+ and intermediate aldehyde dehydrogenase activity
(9–13). CD96 (T cell-activated increased late
expression) is a transmembrane glycoprotein possessing three
extracellular immunoglobulin-like domains (14), which is expressed by T and NK cells
but not the majority of B cells, monocytes and granulocytes in
human peripheral blood cells (15).
Notably however, CD96 has been identified as an LSC-specific marker
in human AML (16,17). Although, the functions and prognosis
value of CD96 expression in human AML remains unclear.
The present study investigated the potential
co-association between CD96 expression and chemotherapy response in
AML, acute lymphoid leukemia (ALL) and mixed lineage acute leukemia
(MAL) patients, to increase the understanding of the role of CD96
in leukemia diagnosis and prognosis.
Materials and methods
Patient samples
BM samples of 105 acute leukemia (AL) patients
presenting with AML, ALL and MAL at the Union Hospital Center for
Stem Cell Research and Application (Wuhan, China) were obtained
following informed consent at diagnosis and following
chemotherapeutic treatment. A total of 15 normal BM samples were
collected as control. The mean age was 48 years and ranged from 2
month to 82 years. The study was approved by the Research Ethics
Committee at the Union Hospital. Patient distributions are shown in
Table I. Diagnosis and identification
of subtypes for the patients was based on morphology using the
French-American-British classification, immune-phenotyping,
molecular genetics and cytogenetics, and fluorescence in
situ hybridization (18). All the
AML patients were induced by a dose-adjusted regimen, except
AML-M3. The complete remission (CR) group was defined as first CR.
When the patient could not achieve the first CR within two
induction treatments, or relapsed in 6 months after the first CR,
they were classified into the non-CR (NCR) group.
 | Table I.Distribution of the patients in the
study. |
Table I.
Distribution of the patients in the
study.
| Classification | No. | Gender, M/F |
|---|
| Normal | 15 |
6/9 |
| AL | 105 |
59/46 |
| AML | 87 |
49/38 |
| M0 | 7 |
5/2 |
| M1 | 10 |
6/4 |
| M2 | 47 |
25/22 |
| M3 | 4 |
1/3 |
| M4 | 10 |
6/4 |
| M5 | 5 |
3/2 |
| M6 | 3 |
2/1 |
| M7 | 1 |
1/0 |
| ALL | 15 |
8/7 |
| MAL | 3 |
2/1 |
Flow cytometry
The majority of the samples were analyzed freshly.
Red blood cells (RBCs) were lysed using a 10 min lysing procedure
on ice with 10 ml lysis buffer [155 mmol/l NH4Cl, 10
mmol/l KHCO3, 0.1 mmol/l Na2 and
ethylenediaminetetraacetic acid (pH 7.4)] and washed with
phosphate-buffered agar (PBA) [phosphate-buffered saline (PBS)
containing 0.1% bovine serum albumin]. The monoclonal antibodies
(10 µl; BD Biosciences, Franklin Lakes, NJ, USA) CD38 fluorescein
isothiocyanate, CD34 cy-chrome and CD96 phycoerythrin were added
respectively to a certain volume of whole BM according to the cell
counts (~5×106 cells/tube). Subsequently the samples
were maintained in the dark at 4°C for 30 min. The samples were
lysed with fluorescence-activated cell sorting (FACS) lysing
solution (BD Biosciences) for 10 min, followed by washing with PBA
once at ~1,000 × g at 4°C for 5 min. Finally, the cell pellets were
resuspended in 300 µl PBS and the data was collected by FACS
Calibur™ (BD Biosciences).
Statistical analysis
The data were analyzed using the SPSS software
package (version 16.0 for Windows; SPSS, Inc., Chicago, IL, USA) in
the study. All the variables were presented as mean values and
standard error (SE) or median and interquartile range with the
independent samples group t-test or χ2 test. Analysis of
the prognosis was performed using the Wilcoxon signed ranks. All
the statistical tests were two-sided and P<0.05 was considered
to indicate a statistically significant difference.
Results
CD34/CD38/CD96 expression in AL
patients
The frequencies of CD34+,
CD34+CD38− and
CD34+CD38−CD96+ populations were
analyzed in BM nucleated cells from a cohort of 105 AL and 15
healthy volunteers. The information of the patients and healthy
volunteers as normal controls is summarized in the Table I. A total of 20,000 nucleated cells
were analyzed for each specimen to evaluate the expression of CD34
and 2,000 gated-CD34+ cells were collected when
analyzing with CD38 and CD96.
As expected, the frequency of the CD34+
population in nucleated cells was low (<4.6%) in all the healthy
volunteers with the mean of 2.07%. However, its expression varied
significantly in AL patients, with the mean value of 35.20% (median
30.26%, SE 0.27%), which was much higher in comparison to the
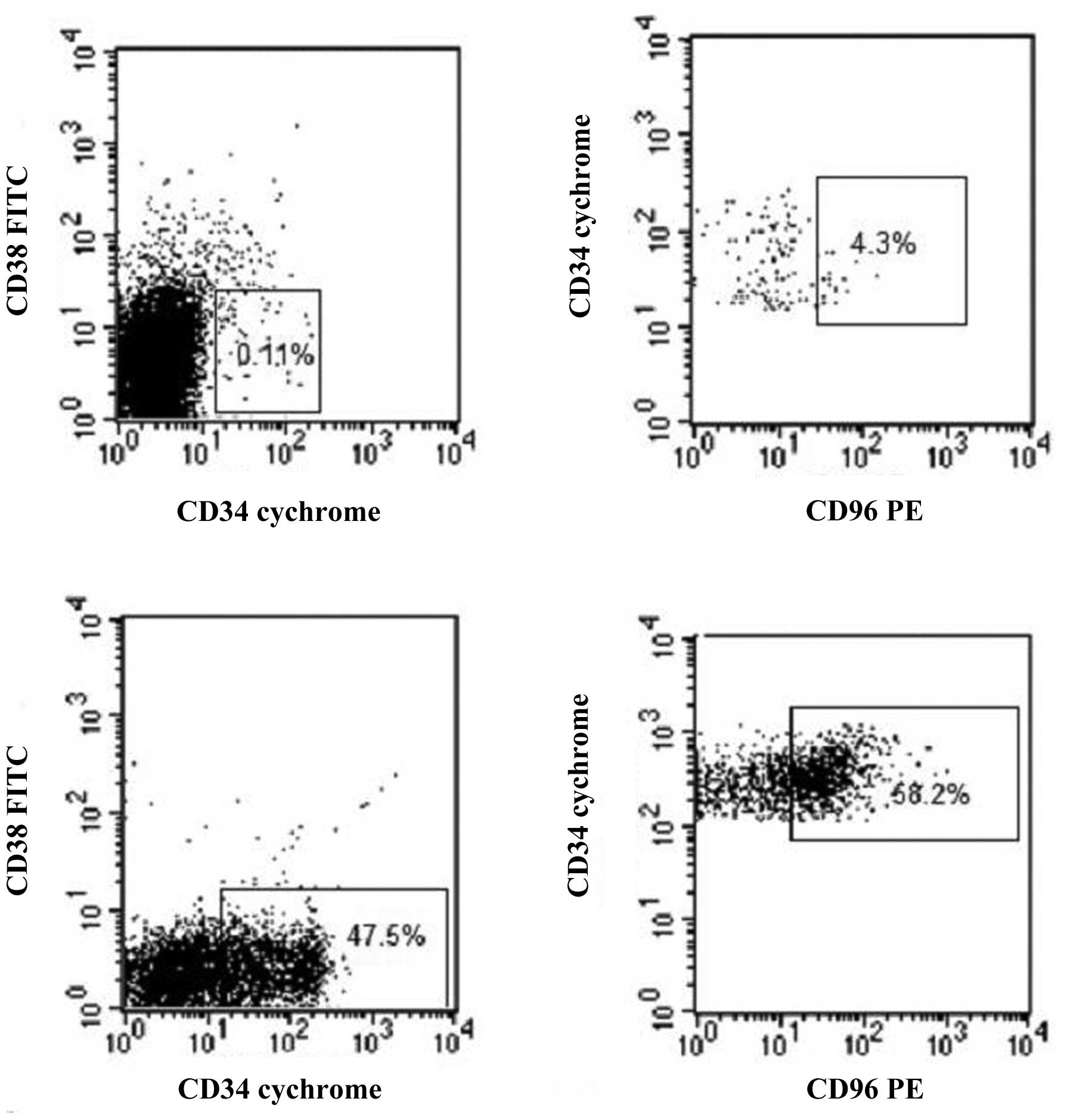
normal controls (P<0.01). Similarly, as shown in Figs. 1 and 2,
the proportion of the CD34+CD38− population
in nucleated cells was <0.6% (mean 0.10%, median 0.03%, SE
0.01%) in all the healthy volunteers, which was 9.22% (median
2.36%, SE 0.14%) in AL patients, indicating the significant
difference between them (P<0.01). Subsequently, the CD96
expression in CD34+CD38− cells was examined.
As shown in Figs. 1 and 3, the CD34+CD38− cells
from healthy volunteers had less expression of CD96, with the mean
of 7.78% (median 4.00%, SE 0.99%), while the proportion was as high
as 29.36% (median 7.32%, SE 0.40%) in the 105 AL patients. The
difference had clear significance (P<0.01).
 | Figure 2.Mean of
CD34+CD38− proportions in nucleated cells in
normal controls and AL patients. The proportion of the
CD34+CD38− population in nucleated cells was
<0.6% (mean 0.10%, median 0.03%, SE 0.01%) in all healthy
volunteers, which was 9.22% (median 2.36%, SE 0.14%) in AL
patients, indicating a significant difference between them
(P<0.01). CD, cluster of differentiation; SE, standard error;
AL, acute leukemia; AML, acute myeloid leukemia; ALL, acute
lymphoid leukemia; MAL, mixed lineage acute leukemia. |
 | Figure 3.Mean of CD96 expression on
CD34+CD38− cells in normal controls and AL
patients. The CD34+CD38− cells from healthy
volunteers expressed CD96 less, with the mean of 7.78% (median
4.00%, SE 0.99%), while the proportion was as high as 29.36%
(median 7.32%, SE 0.40%) in 105 AL patients. The difference was
statistically significant (P<0.01). CD, cluster of
differentiation; SE, standard error; AL, acute leukemia; AML, acute
myeloid leukemia; ALL, acute lymphoid leukemia; MAL, mixed lineage
acute leukemia. |
The CD34 expression also varied significantly in AML
patients, with the mean value of 35.12% (median 27.80%, SE 0.32%),
which was significantly higher compared to the healthy controls
(P<0.01), but was close to the total AL samples. Similarly, the
proportion of the CD34+CD38− population in
nucleated cells in healthy volunteers was much lower compared to
the AML patients (P<0.01), which was at the mean of 6.91%
(median 1.25%, SE 0.15%). In addition, CD96 expression on
CD34+CD38− cells in AML patients (mean
26.71%, median 5.57%, SE 0.33%) was also significantly higher
compared to the normal control (P<0.01).
The CD34 expression in ALL patients was at the mean
value of 39.15% (median 35.03%, SE 2.33%), which was significantly
higher compared to the healthy controls (P<0.01), but there was
no difference when comparing with the AML patients (P>0.05). The
CD34+CD38− cells in the ALL patients was at
the mean value of 24.48% (median 23.22%, SE 1.44%), which was
significantly higher compared to the healthy controls (P<0.01),
but still much lower than the AML patients (P<0.01). The CD96
expression in the CD34+CD38− cells in the ALL
patients (mean 11.34%, median 0.74%, SE 1.86%) was similar to the
normal controls (P>0.05) and lower than the AML patients
(P<0.05). With regards to MAL, further analysis or comparisons
were not performed due to the limited cases.
CD34/CD38/CD96 expression in the AML
subtypes
The differences between 3 subtypes of AML (M1, M2,
and M4) were considered, which had ≥10 samples in each group. Only
the CD34 expression in M2 and M4 patients had a statistical
difference (P<0.05). None of the other indices, including
CD34+ cell percentages in the M1 and M2 or M1 and M4
groups, CD34+CD38− proportions and CD96
expression in the 3 subtypes, were identified as significantly
difference (P>0.05).
Association of
CD34+CD38− and CD96+ expression
with the chemotherapy response in AML patients
The presence of LSCs has been proposed to be an
important reason for drug resistance in AML patients. Thus, the
potential co-association between the CR rates and frequencies of
LSC-enriched populations were examined in the AML patients. A total
of 55 AML patients with necessary clinical information to evaluate
chemotherapy response were included in the analysis. The overall CR
rate in this cohort of patients was 69.1% (38/55).
These patients were divided into two groups
according to the frequencies of the
CD34+CD38− population in nucleated cells. The
results showed that in 17 NCR patients, the
CD34+CD38− proportion was at the mean of
2.31% (median 0.23%, SE 0.28%), which was different from that in 38
CR patients with the mean of 8.19% (median 1.97%, SE 0.34%)
(P<0.05). However, further data suggested that all 10 cases of
AML patients with >15% CD34+CD38− cells
achieved CR, while 17 out of 45 patients (38%) who had <15%
CD34+CD38− cells remained NCR (P<0.01), as
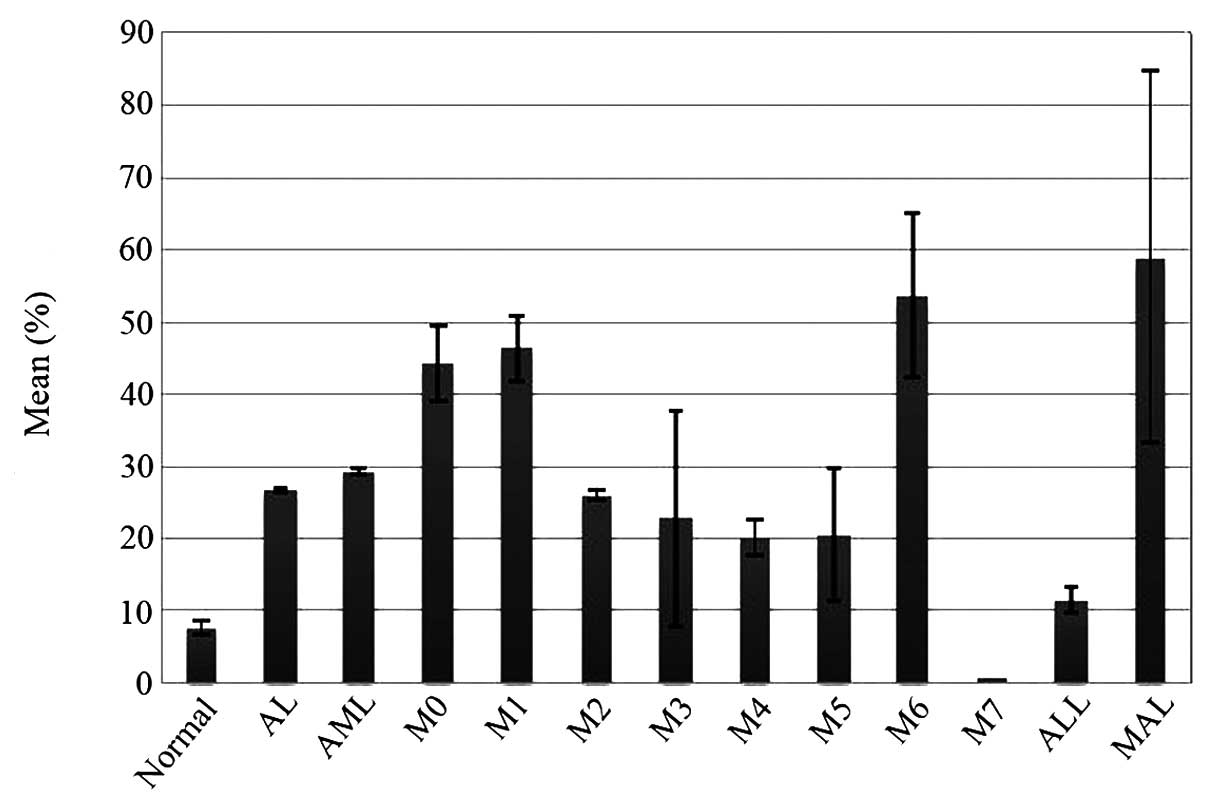
shown in Fig. 4 and Table II. Therefore, all the 17 NCR patients
had <15% CD34+CD38− cells in nucleated
cells.
 | Figure 4.Mean of
CD34+CD38− and CD96+ proportions
in newly diagnosed AML patients who achieved CR or NCR. The results
showed that in 17 NCR AML patients, the average
CD34+CD38− proportion was at the mean of
2.31% (median 0.23%, SE 0.28%), which was different from that in 38
CR patients with the mean of 8.19% (median 1.97%, SE 0.34%)
(P<0.05). However, there was no difference in the mean of CD96
expression on the CD34+CD38− fractions
between the two groups of AML patients (P>0.05). CD, cluster of
differentiation; SE, standard error; AML, acute myeloid leukemia;
CR, complete remission; NCR, non CR. |
 | Table II.CD34+CD38−
proportions and the chemotherapy response of AML patients. |
Table II.
CD34+CD38−
proportions and the chemotherapy response of AML patients.
|
CD34+CD38−cells in
CD34+ cells | NCR cases | CR cases | Subtotal |
|---|
| ≥15% | 0 | 10 | 10 |
| <15% | 17 | 28 | 45 |
| Subtotal | 17 | 38 | 55 |
Subsequently, whether CD96 expression in the
CD34+CD38− cells was correlated with drug
resistance in AML patients was examined. The results showed there
was no difference in the CD96+ proportion between the 17
NCR patients at the mean of 30.09% and 38 CR patients at the mean
of 22.09% (P>0.05). Of note, 65% NCR patients had >10%
CD34+CD38−CD96+ population in the
CD34+CD38− cells, while only 32% CR patients
had the same level of
CD34+CD38−CD96+ population
(P<0.01). Therefore, 11 out of 23 patients (48%) with >10%
CD96 expression remained NCR, while the rate in patients with
<10% CD96 cells was only 19% (P<0.01). The results are shown
in Fig. 4 and Table III.
 | Table III.CD96 expression in
CD34+CD38− cells and the response for
chemotherapy of AML patients. |
Table III.
CD96 expression in
CD34+CD38− cells and the response for
chemotherapy of AML patients.
|
CD34+CD38−CD96+cells
in CD34+CD38− cells | NCR cases | CR cases | Subtotal |
|---|
| ≥10% | 11 | 12 | 23 |
| <10% | 6 | 26 | 32 |
| Subtotal | 17 | 38 | 55 |
Discussion
CD96 had previously been reported to express on T
and NK cells, but not on B cells, granulocytes, monocytes or RBCs
(17,19). However, recent studies suggested that
CD96 was a putative maker expressed by LSCs in AML patients.
Although the physiological functions of CD96 on AML-LSCs are
unknown, it may contribute to their adhesion to the BM compartment.
To increase the understanding of the association of CD96 expression
and CD34+CD38− stem cell markers and
subsequently to reveal the role of CD96 in leukemia, the
CD34/CD38/CD96 expression and the clinical characteristics in 105
AL patients, including 87 AML, 15 ALL and 3 MAL, and 15 healthy
volunteers as normal controls were examined.
As expected, the frequency of the CD34+
population in nucleated cells was much lower than that in the AL
patients (P<0.01), which was in the same situation as
CD34+CD38− and
CD34+CD38−CD96+ expression
(P<0.01), as shown in Figs.
1–3. Advanced analysis also
showed that AML and ALL had higher CD34+ and
CD34+CD38− cells compared to the normal
controls (P<0.01). These results were consistent with previous
studies (13,20,21).
However, the present data also showed that although there were high
frequencies and no difference in CD34+ expression
between AML and ALL (P>0.05), the
CD34+CD38− cells in ALL patients was
significantly lower than that in the AML patients (P<0.01). In
addition, there were still evident differences of CD96 expression
on CD34+CD38− cells in ALL patients with that
in AML; the former was similar to the normal controls (P>0.05)
and lower than the AML patients (P<0.05). The results suggested
that, regardless of the several studies indicating a population of
LSC exhibiting a CD34+CD38− phenotype in ALL
and AML (22,23), the proportions were different. CD96
may not appear to be a potential distinguished LSCs marker in ALL.
However, except for the statistical difference of CD34 expression
in the M2 and M4 patients (P<0.05), none of the other indices,
including the CD34+ cell percentages in the M1 and M2 or
M1 and M4 groups, CD34+CD38− proportions and
CD96 expression in the 3 subtypes of AML (M1, M2 and M4), were
identified as different (P>0.05). Sufficient cases may be
studied to evaluate the diversity.
Numerous studies were performed to reveal the
characteristics and function of leukemia stem cells. One of the
most prevalent aims focused on the CD34+CD38−
LSC-enriched cells, which had been proposed as an important factor
in drug resistance. Certain studies described that the fraction of
CD34+CD38− cells at the time of diagnosis
exhibited a significant correlation with poor prognosis in
childhood ALL of B-cell lineage and AML (20,21). Of
note, the present results showed that in 17 NCR patients out of 55
AML cases, the mean of CD34+CD38− proportion
in CD34+ cells at the new diagnosis time was lower
compared to the 38 CR AML patients (P<0.05). Furthermore, all 10
cases of AML patients with >15% CD34+CD38−
cells achieved CR, while 17 out of 45 patients (38%) who had
<15% CD34+CD38− cells remained NCR
(P<0.01), as shown in Fig. 4 and
Table II. In summary, all 17 NCR
patients had <15% CD34+CD38− cells in
nucleated cells. However, conflicting results were not identified
between our and the previous study. The previous studies analyzed
the fraction of CD34+CD38− cells based on
total abnormal cells, which may or may not include a significant
number of CD34+ cells. The
CD34+CD38− cells were counted based on pure
CD34+ cells, which varied significantly in all the AL
patients even in a similar proportion of abnormal cells, but may be
more comparable. This type of percentage was selected as it may
avoid the inaccuracy of too few CD34+CD38−
cells for the rare CD34+ cells, and waive the difference
resulting from the unbalance of various amounts of CD34+
cells in the abnormal cells. The frequencies should not simply be
compared. The CD34+CD38− proportion in the
CD34+ cells could also provide a significant explanation
for the prognosis of AL. In addition, the results verified that
only the CD34+CD38− cells were an enriched
marker of LSCs. The amount of LSCs may not be positively correlated
with the CD34+CD38− proportion in
CD34+ cells, or conversely, lower
CD34+CD38− frequencies (<15%) in
CD34+ cells may suggest more non-developed LSCs. These
results indicated that AML-LSC could be distinguished from normal
HSC by the presence of CD96 expression. This finding suggested that
CD96 may be an excellent candidate target for antibody therapy
against LSC. The therapy may be developed by the CD96 antibodies
that induce cytotoxicity, such as antibody-dependent cell-mediated
cytotoxicity, augmented macrophage phagocytosis or complement
dependent cytotoxicity (24). Further
research should focus on identifying the real LSCs.
Subsequently, the mean CD96+ proportion
between 17 NCR patients and 38 CR patients was compared and there
was no difference (P>0.05). Notably, as shown in Fig. 4 and Table
III, the data showed that 65% of NCR patients had >10%
CD34+CD38−CD96+ population in
CD34+CD38− cells, while only 32% CR patients
had the same level of
CD34+CD38−CD96+ population
(P<0.01). In summary, 11 out of 23 patients (48%) with >10%
CD96 expression remained NCR, while the rate in patients with
<10% CD96 cells was only 19% (P<0.01). The results strongly
indicated that a higher expression of CD96 (>10%) may promote a
poor response for chemotherapy, which may be closely associated
with primary resistant. Of note, CD96 was proved to be an efficient
identical marker of LSCs in CD34+CD38−
groups, which was consistent with previous studies (16,17). For
the limit of adherence and follow-up of the patients, only the
outcomes in 55 AML patients with completed clinical data were
analyzed, however, it may have a certain association with other
types or subtypes of leukemia that require further research.
CD96 expression was also evaluated in
CD34+CD38− cells in 14 high-risk MDS BM
samples. Although CD96 expression was much higher compared to the
normal control, no evidence showed that the
CD34+CD38− or
CD34+CD38−CD96+ proportion was
associated with MDS chemotherapy efficacy or the prognosis.
However, MDS stem cells exhibit a deranged phenotype that is
different from normal and AML stem cells, and this may cause them
to be particularly difficult to eradicate by therapies targeted
against surface antigens.
In conclusion, CD96 was frequently expressed in the
CD34+CD38− LSC population in AL patients.
CD96 is significantly associated with the response for chemotherapy
in AML patients, which strongly suggested that CD96 may be a marker
of LSCs, candidate therapeutic target and prediction factor in AL
patients.
Acknowledgements
The authors acknowledge the Union Hospital and
Center for Stem Cell Research and Application for supporting the
research. The present study was supported by the Morning Program,
Wuhan Science and Technology Bureau (grant no. 201150431122) and
the National Young Scientist's Program of China (grant no.
81100356).
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Passegué E, Jamieson CH, Ailles LE and
Weissman IL: Normal and leukemic hematopoiesis: Are leukemias a
stem cell disorder or a reacquisition of stem cell characteristics?
Proc Natl Acad Sci USA. 100:(Suppl 1). 11842–11849. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warner JK, Wang JCY, Hope KJ, Jin L and
Dick JE: Concepts of human leukemic development. Oncogene.
23:7164–7177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huntly BJ and Gilliland DG: Leukaemia stem
cells and the evolution of cancer-stem-cell research. Nat Rev
Cancer. 5:311–321. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weissman I: Stem cell research: Paths to
cancer therapies and regenerative medicine. JAMA. 294:1359–1366.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Costello R, Mallet F, Chambost H, Sainty
D, Arnoulet C, Gastaut JA and Olive D: The immunophenotype of
minimally differentiated acute myeloid leukemia (AML-M0): Reduced
immunogenicity and high frequency of
CD34+/CD38− leukemic progenitors. Leukemia.
13:1513–1518. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Rhenen A, Feller N, Kelder A, Westra
AH, Rombouts E, Zweegman S, van der Pol MA, Waisfisz Q,
Ossenkoppele GJ and Schuurhuis GJ: High stem cell frequency in
acute myeloid leukemia at diagnosis predicts high minimal residual
disease and poor survival. Clin Cancer Res. 11:6520–6527. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malaise M, Steinbach D and Corbacioglu S:
Clinical implications of c-Kit mutations in acute myelogenous
leukemia. Curr Hematol Malig Rep. 4:77–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wakita S, Yamaguchi H, Miyake K, Mitamura
Y, Kosaka F, Dan K and Inokuchi K: Importance of c-kit mutation
detection method sensitivity in prognostic analyses of
t(8;21)(q22;q22) acute myeloid leukemia. Leukemia. 25:1423–1432.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SH, Chi HS, Min SK, Park BG, Jang S
and Park CJ: Prognostic impact of c-KIT mutations in core binding
factor acute myeloid leukemia. Leuk Res. 35:1376–1383. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chao MP, Alizadeh AA, Tang C, Jan M,
Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL and Majeti R:
Therapeutic antibody targeting of CD47 eliminates human acute
lymphoblastic leukemia. Cancer Res. 71:1374–1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gerber JM, Smith BD, Ngwang B, Zhang H,
Vala MS, Morsberger L, Galkin S, Collector MI, Perkins B, Levis MJ,
et al: A clinically relevant population of leukemic CD34(+)CD38(–)
cells in acute myeloid leukemia. Blood. 119:3571–3577. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meyer D, Seth S, Albrecht J, Maier MK, du
Pasquier L, Ravens I, Dreyer L, Burger R, Gramatzki M, Schwinzer R,
et al: CD96 interaction with CD155 via tts first Ig-like domain is
modulated by alternative splicing or mutations in distal Ig-like
domains. J Biol Chem. 284:2235–2244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuchs A, Cella M, Giurisato E, Shaw AS and
Colonna M: Cutting edge: CD96 (tactile) promotes NK cell-target
cell adhesion by interacting with the poliovirus receptor (CD155).
J Immunol. 172:3994–3998. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosen N, Park CY, Tatsumi N, Oji Y,
Sugiyama H, Gramatzki M, Krensky AM and Weissman IL: CD96 is a
leukemic stem cell-specific marker in human acute myeloid leukemia.
Proc Natl Acad Sci USA. 104:11008–11013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gramatzki M, Ludwig WD, Burger R, Moos P,
Rohwer P, Grünert C, Sendler A, Kalden JR, Andreesen R, Henschke F,
et al: Antibodies TC-12 (‘unique’) and TH-111 (CD96) characterize
T-cell acute lymphoblastic leukemia and a subgroup of acute myeloid
leukemia. Exp Hematol. 26:1209–1214. 1998.PubMed/NCBI
|
|
18
|
Löwenberg B, Downing JR and Burnett A:
Acute myeloid leukemia. N Engl J Med. 341:1051–1062. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang PL, O'Farrell S, Clayberger C and
Krensky AM: Identification and molecular cloning of tactile. A
novel human T cell activation antigen that is a member of the Ig
gene superfamily. J Immunol. 148:2600–2608. 1992.PubMed/NCBI
|
|
20
|
Ebinger M, Witte KE, Ahlers J, Schӓfer I,
André M, Kerst G, Scheel-Walter HG, Lang P and Handgretinger R:
High frequency of immature cells at diagnosis predicts high minimal
residual disease level in childhood acute lymphoblastic leukemia.
Leuk Res. 34:1139–1142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Witte KE, Ahlers J, Schӓfer I, André M,
Kerst G, Scheel-Walter HG, Schwarze CP, Pfeiffer M, Lang P,
Handgretinger R, et al: High proportion of leukemic stem cells at
diagnosis is correlated with unfavorable prognosis in childhood
acute myeloid leukemia. Pediatr Hematol Oncol. 28:91–99. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castor A, Nilsson L, Astrand-Grundström I,
Buitenhuis M, Ramirez C, Anderson K, Strömbeck B, Garwicz S,
Békássy AN, Schmiegelow K, et al: Distinct patterns of
hematopoietic stem cell involvement in acute lymphoblastic
leukemia. Nat Med. 11:630–637. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong D, Gupta R, Ancliff P, Atzberger A,
Brown J, Soneji S, Green J, Colman S, Piacibello W, Buckle V, et
al: Initiating and cancer-propagating cells in TEL-AML1-associated
childhood leukemia. Science. 319:336–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams GP and Weiner LM: Monoclonal
antibody therapy of cancer. Nat Biotechnol. 23:1147–1157. 2005.
View Article : Google Scholar : PubMed/NCBI
|