Introduction
Acute leukemia and non-Hodgkin lymphoma (NHL) are
the most common malignancies in children and adolescents (1,2), with an
increasing incidence observed in children and adults worldwide over
the last few decades (3–5). Although ionizing radiation, certain
chemotherapeutic agents, certain infections, a family history of
disease and certain genetic disorders have been reported to be
associated with hematological malignancies, the etiology of acute
leukemia and NHL has not been fully elucidated.
Severe autoimmune conditions, such as systemic lupus
erythematosus and rheumatoid arthritis, have been found to be
associated with acute leukemia and NHL; however, little is known on
the role of subtle immune impairments in the development of
diseases, such as asthma. Asthma is an aberrant chronic immune
response due to a lack of infections during early childhood
(6,7).
The association of asthma with acute leukemia and NHL may be based
on two hypotheses: One is the immune surveillance hypothesis,
according to which the possible mechanism underlying the reduced
the risk of asthma patients to develop acute leukemia and NHL is
that asthma enhances the ability of the immune system to detect and
destroy cancer cells (8). By
contrast, the antigenic stimulation hypothesis suggests that
immune-stimulating conditions may predispose to cancer, due to an
increasing number of random mutations in activated dividing immune
cells (9–12). Previous studies suggested that asthma
was protective against leukemia and lymphoma (13–16),
whereas other studies indicated asthma as a risk factor (17–19), or
reported no association (20–23).
Given the conflicting results on the association of
asthma with the risk of leukemia and NHL, a meta-analysis was
conducted to elucidate this association. The objective of the
present study was to evaluate the potential association between a
history of asthma and the incidence of acute leukemia and NHL.
Materials and methods
Literature search
Two authors independently performed a literature
search through PubMed and the Cochrane Database of Systematic
Reviews through to April 20, 2014. The terms used in the search
were ‘asthma AND (leukemia or lymphoma)’. The title and abstract of
every article were reviewed. According to the inclusion criteria,
full-text articles were selected. The reference lists of the
full-text articles were also searched for additional relevant
studies.
Inclusion and exclusion criteria
An article was considered as useful if it reported
original data on the association between asthma and the risk of
acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) or
NHL from epidemiological studies. Only studies published in English
were included. The articles included were required to provide
sufficient information to calculate the odds ratio (OR). Any
disagreements regarding inclusion or exclusion of a study were
resolved by a joint review with other authors. Only the most recent
articles were selected when the same study produced several
publications.
Data extraction
Two authors independently performed the data
extraction. The extracted data included name of first author,
publication year, country, time of diagnosis of acute leukemia or
NHL, age range of cases, number of samples (cases, controls,
asthmatics and non-asthmatics), methods of assessment of asthma
(medical records or interview questionnaires) and matching factors.
Any disagreements were resolved by a joint assessment of the
original study with other authors.
Quality assessment
Two authors independently evaluated the quality of
the selected studies using the Newcastle-Ottawa Scale (NOS). The
NOS for case-control studies includes three parameters, namely
selection, comparability and exposure assessment. Any individual
article may get the maximal NOS score of 4 points for selection, 2
for comparability and 3 for exposure. A NOS score of 1–3 was
considered as low-quality, a score of 4–6 as intermediate-quality
and a score of 7–9 as high-quality. Any disagreements on the NOS
score of a study were resolved by a joint review of the original
article.
Data synthesis and analysis
The primary result measured was OR with 95%
confidence interval (CI) of developing ALL, AML or NHL in patients
with a history of asthma. The Mantel-Haenszel method and the
random-effects model were applied to measure the outcomes (24,25). The
I2 index was used to evaluate heterogeneity (26). I2 values of 25, 50 and 75%
were considered to reflect mild, moderate and severe heterogeneity,
respectively. Meta-analyses were performed for ALL, AML and NHL
using the Review Manager 5.2 software (Copenhagen: The Nordic
Cochrane Centre, The Cochrane Collaboration, 2012). In the forest
plots, OR values >1 suggested an increased risk, whereas OR
values <1 suggested a decreased risk of cases among individuals
with a history of asthma.
Results
Search results
The results of the literature search are summarized
in Fig. 1. A total of 668 records
were identified during our initial search. Following a review of
the titles and abstracts, 618 articles did not pertain to the
association between asthma and the risk of acute leukemia or NHL
and were excluded. The remaining 50 articles were retrieved for
full-text review. Following a detailed review, a further 34
articles were excluded (27 were irrelevant to the association
between asthma and the incidence of acute leukemia or NHL, 1 was a
case report and 6 were reviews). No additional articles were
identified through searching the reference lists of the 50
articles. Finally, 16 case-control studies were included in our
meta-analysis.
Characteristics of the studies
The main characteristics of the 16 studies included
in our analysis are summarized in Table
I. The studies were published between 1996 and 2012. A total of
7 studies originated from Europe, 6 from North America, 2 from
Australia and 1 from Asia. The time of diagnosis of the cases was
between 1980 and 2008. The cases in the studies were children or
adolescents in 9 studies, adults in 6 studies and both in 1 study.
A total of 5,738 ALL patients, 4,362 AML patients, 22,048 NHL
patients and 168,397 controls were included in our analysis. A
history of asthma was identified by interviewing with
questionnaires in 12 studies, reviewing medical records in 3
studies and using both methods in 1 study. In all the studies, the
controls were matched to the patients by age and gender and 8
studies were adjusted by region of residence. As regards quality
assessment, all the included studies were of high quality (NOS
score ≥7).
 | Table I.Characteristics of the 16 case-control
studies included in the meta-analysis of the asociation of asthma
with acute leukemia and non-Hodgkin lymphoma. |
Table I.
Characteristics of the 16 case-control
studies included in the meta-analysis of the asociation of asthma
with acute leukemia and non-Hodgkin lymphoma.
| First author, year
(Refs.) | Country | Time of
diagnosis | Age range, years | No. of cases | No. of controls | Assessment of
asthma | Matching factors | NOS score |
|---|
| Chang, 2012 (17) | Taiwan | 2000–2008 | 1–10 | 846 ALL | 3,674 | Medical records | Gender, birth date,
diagnosis time | 8 |
| Cooper, 1996
(20) | USA/Canada | 1986–1990 | 18–79 | 121 ALL, 622 AML | 637 | Interview | Age, gender, race,
residence | 8 |
| Cozen, 2007 (22) | USA | 1998–2000 | 20–74 | 604 NHL | 464 | Interview | Age, gender | 8 |
| Dikalioti, 2012
(15) | Greece | 1996–2008 | 0–14 | 166 NHL | 166 | Interview | Age, gender | 7 |
| Grulich, 2005
(27) | Australia | 2000–2001 | 20–74 | 699 NHL | 691 | Interview | Age, gender,
residence | 8 |
| Hughes, 2007
(19) | UK | 1991–1996 | ≤14 | 720 ALL, 101 AML | 1,337 | Interview and medical
records | Age, gender,
residence | 7 |
| Jourdan-Da Silva,
2004 (13) | France | 1995–1998 | ≤15 | 400 ALL, 63 AML | 565 | Interview | Age, gender,
residence | 8 |
| Lee, 2004 (28) | USA | 1980–1986 | ≥21 | 872 NHL | 2,336 | Interview | Race, age, gender,
vital status | 8 |
| Lee, 2006 (29) | USA | 1998–2000 | 20–74 | 668 NHL | 543 | Interview | Age, gender, race,
residence | 8 |
| Rudant, 2010
(14) | France | 2003–2004 | 1–15 | 633 ALL, 86
AML | 1,493 | Interview | Age, gender | 8 |
| Rudant, 2011
(30) | France | 2003–2004 | 2–14 | 164 NHL | 1,311 | interview | Age, gender | 8 |
| Schuz, 2003
(31) | Germany | 1980–1994 | ≤14 | 996 ALL | 2,441 | Interview | Age, gender,
residence | 8 |
| Soderberg, 2006
(21) | Sweden | 1987–1999 | 0–103 | 18,186 NHL, 3,490
AML | 149,344 | Medical
records | Age, gender, year
of diagnosis | 9 |
| Spector, 2004
(18) | USA | 1985–1999 | 0.23–6.86 | 180 ALL | 718 | Medical
records | Age, gender,
diagnosis time | 8 |
| Vajdic, 2007
(32) | Australia | 2000–2001 | 20–74 | 689 NHL | 691 | Interview | Age, gender,
residence | 8 |
| Wen, 2000 (33) | USA | 1989–1993 | ≤15 | 1,842 ALL | 1,986 | Interview | Age, gender, race,
residence | 8 |
Outcome results
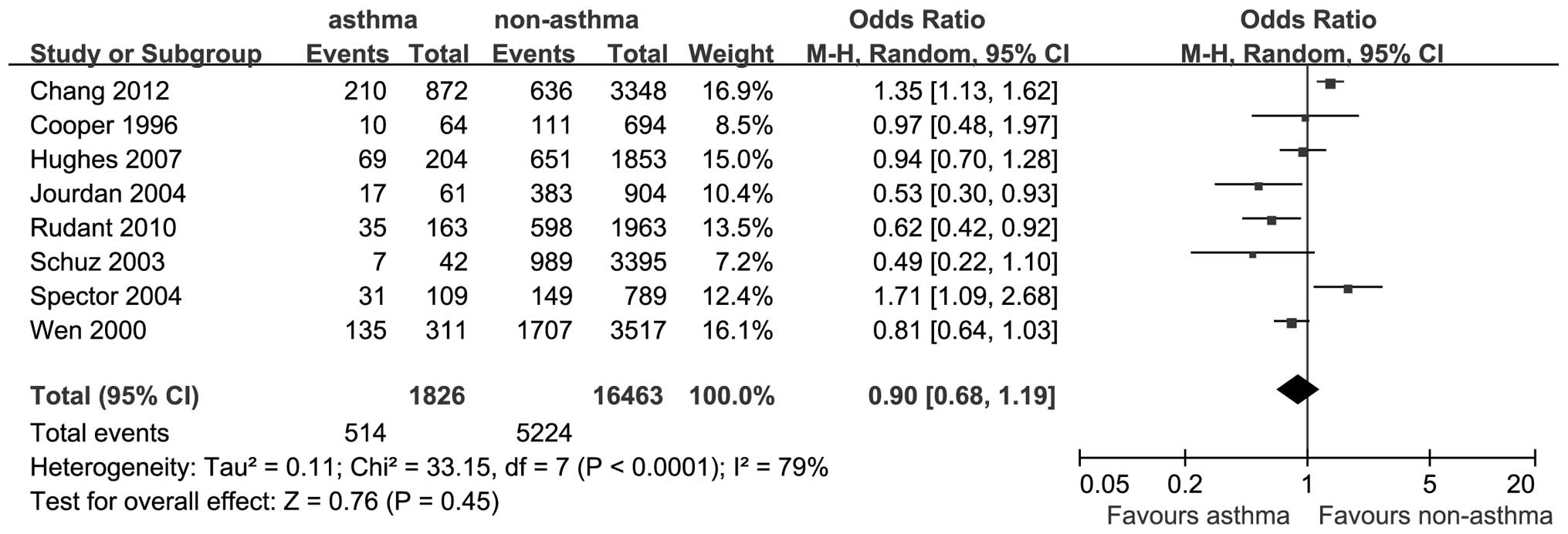
A total of 8 studies reported outcomes on the
association between asthma and the risk of ALL. The OR for ALL was
0.90 (95% CI: 0.68–1.19; P=0.45; I2=79%; Fig. 2).
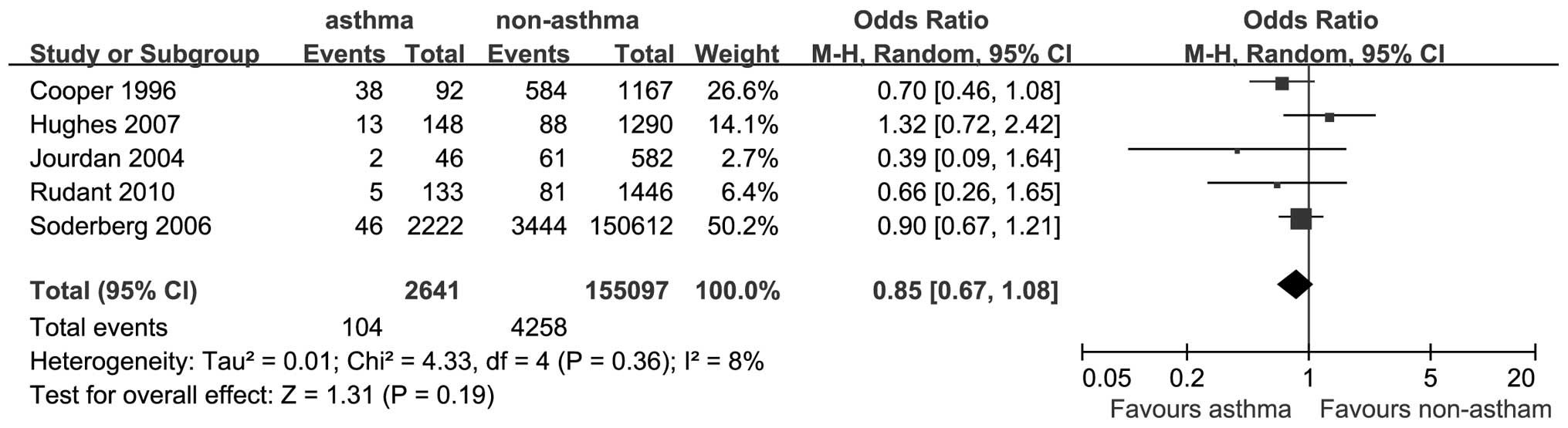
A total of 5 studies provided data on the
association between asthma and the risk of AML. The OR for AML was
0.85 (95% CI: 0.67–1.08; P=0.19; I2=8%; Fig. 3).
A total of 8 studies reported data on the
association between asthma and the risk of NHL. The OR for NHL was
0.91 (95% CI: 0.83–1.00; P=0.05; I2=0%; Fig. 4).
There was a significantly reduced risk of developing
AML in asthmatic patients. In addition, there was an inverse
association between asthma and acute leukemia, but this association
was not statistically significant.
Discussion
In our meta-analysis, asthma was found to be
associated with statistically significant reductions in the risk of
NHL, whereas it was associated with non-statistically significant
reductions in the risk of ALL and AML.
A hypothesis has been proposed with regard to the
mechanism of the inverse association of asthma with NHL. According
to the cancer immune surveillance hypothesis, the immune system
identifies and eradicates aberrant cells in the body, thus averting
the development of a number of potential malignancies (34). The occurrence of asthma may be a
surrogate marker of an increased ability of the immune system to
recognize and destroy malignant cells (23). By contrast, the antigenic stimulation
hypothesis suggests that the chronic inflammatory state in
asthmatic patients may result in an inflammatory cytokine cascade
and lead to tissue damage and, ultimately, lymphoid malignancies
(35). Asthma was associated with a
reduced risk of NHL, which supports the immune surveillance
hypothesis.
Our results revealed a non-significant odds ratio of
0.90 and 0.85 for the association of a history of asthma with ALL
and AML, respectively,. The literature on asthma and acute leukemia
is heterogeneous. In the present analysis, a ~10% reduction in the
risk for NHL in patients with a history of asthma was observed. An
IgE-mediated anticancer response in asthma patients may be a
possible explanation for the protective effect of asthma against
the development of NHL (22). In
addition, histamine, an inflammatory mediator in asthma, was
reported to have the ability to defend normal cells by suppressing
oxygen radical-induced damage and activating protective lymphocytes
(36).
Asthma is considered as an overactive state of the
immune response, characterized by a shift towards a Th2-dominant
immunity. The persistence of a Th2-dominant immune system in
asthmatic patients is inversely associated with the risk of NHL, of
which the mechanism has not been fully elucidated. Other unmeasured
factors associated with asthma may contribute to the reduced risk
of NHL. Elucidating the mechanism of this negative association may
provide valuable information on the effect of asthma on the immune
response and the subsequent risk of developing NHL. The true
underlying mechanism was not determined in the present analysis;
however, our results support the immune surveillance hypothesis. We
also hypothesize that genetic or exposure factors underlying the
development of asthma may also be protective against NHL.
When analyzing the association between asthma and
ALL, a high degree of heterogeneity was observed among the studies.
A possible explanation for this severe heterogeneity may be the
differences in the included studies regarding age range, country,
number of cases and controls, diagnosis time and matching factors.
Major differences in baseline characteristics largely account for
the heterogeneity. By contrast, only mild heterogeneity and no
heterogeneity were observed in the analysis of AML and NHL,
respectively. Consequently, the results of the inverse association
between asthma and NHL may be considered as credible.
The results of the meta-analysis should be
interpreted with caution due to certain limitations. First, in the
majority of the studies, the history of asthma was obtained from
interview by telephone or questionnaire and recall bias may exist
in the self-reported data (37,38).
Although the information bias appeared to be inevitable, the
inverse association of asthma with acute leukemia and NHL
(significant in NHL and non-significant in ALL and AML) was
notable, provided that patients are more likely to pay attention to
disease history, leading to information bias of a false-positive
association (27). The positive
association between asthma and the risk of developing acute
leukemia or NHL was reported in previous studies (17,18).
Shared etiological factors may account for the results. However, as
the reference date, corresponding with the date of diagnosis in
cancer patients, is not well-defined in the controls, a history of
asthma may be over-reported (31).
This may lead to a higher incidence of asthma in the controls, from
whom the data were obtained by interview. Previous studies reported
only a moderate consistency between the medical reports and
interview data (19). Second, there
was no sufficient information on the morphological, immunological,
genetic, molecular or pathological subtypes of acute leukemia and
NHL in previous studies; therefore, further analysis of the
association between asthma and the subtypes of these diseases was
not feasible. Third, there was also a possible information bias in
the data based on the medical records, as less severe cases of
asthma may be not documented in medical records. This
misclassification may exist equally among cases and controls, or
more frequently in controls, resulting in a positive association
trend of asthma with acute leukemia and NHL. Fourth, asthma-related
medications may modulate the immune system, affecting the
association of asthma with acute leukemia and NHL. The medical
treatment of asthma may affect the risk of developing cancer due to
cellular and humoral immunosuppression. In addition, the duration
and severity of asthma, should also be taken into consideration.
Fifth, confounding factors including age, gender and region of
residence were controlled in the majority of the included studies,
but other confounding factors, such as genetic characteristics,
individual lifestyle, other immune-related diseases and infection
exposure cannot be completely ruled out.
There were also several strengths in our study.
First, the number of included cases and controls was sufficiently
large to obtain a reliable association between asthma and ALL, AML
and NHL. Second, the studies in our analysis included a diversity
of countries and races, enabling the generalization of the
outcomes. Third, with regard to NOS, all the studies included in
our analysis were of high quality, ensuring reliable results.
Fourth, in all the included studies, the cases and controls were
matched by age and gender; region of residence and race were also
matched in some studies, minimizing the potential information bias
between cases and controls. Fifth, in the majority of the studies,
a history of asthma was identified shortly before the diagnosis of
acute leukemia or NHL. Therefore, asthma during the early or
indolent course of these diseases was excluded.
In conclusion, this study demonstrated that asthma
is inversely associated with the incidence of NHL. A negative trend
in the risk of ALL and AML in subjects with a history of asthma was
also observed. Additional large prospective studies are required to
elucidate the association between asthma and hematological
malignancies.
References
|
1
|
Curado MP, Edwards B and Shin HR: Cancer
incidence in five continents. 9. IARC Scientific Publications;
Lyon: 2007
|
|
2
|
Stiller CA and Parkin DM: Geographic and
ethnic variations in the incidence of childhood cancer. Br Med
Bull. 52:682–703. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weidmann C, Black RJ, Masuyer E and Parkin
DM: Incidence of non-Hodgkin's lymphoma in children between 1970
and 1990 in nine European countries. Eur J Cancer. 35:1235–1237.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Izarzugaza MI, Steliarova-Foucher E,
Martos MC and Zivkovic S: Non-Hodgkin's lymphoma incidence and
survival in European children and adolescents (1978–1997): report
from the Automated Childhood Cancer Information System project. Eur
J Cancer. 42:2050–2063. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parker DM, Whelan SL, Ferlay J, Raymond L
and Young J: Cancer incidence in five continents. 7. IARC
Scientific Publications; Lyon: 2007
|
|
6
|
Greaves M: Infection, immune responses and
the aetiology of childhood leukaemia. Nat Rev Cancer. 6:193–203.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Urayama KY, Buffler PA, Gallagher ER,
Ayoob JM and Ma X: A meta-analysis of the association between
day-care attendance and childhood acute lymphoblastic leukaemia.
Int J Epidemiol. 39:718–732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Severson RK, Davis S, Thomas DB, Stevens
RG, Heuser L and Sever LE: Acute myelocytic leukemia and prior
allergies. J Clin Epidemiol. 42:995–1001. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gallagher RP, Spinelli JJ, Elwood JM and
Skippen DH: Allergies and agricultural exposure as risk factors for
multiple myeloma. Br J Cancer. 48:853–857. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bernard SM, Cartwright RA, Bird CC,
Richards ID, Lauder I and Roberts BE: Aetiologic factors in
lymphoid malignancies: a case-control epidemiological study. Leuk
Res. 8:681–689. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McWhorter WP: Allergy and risk of cancer.
A prospective study using NHANESI followup data. Cancer.
62:451–455. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doody MM, Linet MS, Glass AG, et al:
Leukemia, lymphoma and multiple myeloma following selected medical
conditions. Cancer Causes Control. 3:449–456. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jourdan-Da Silva N, Perel Y, Mechinaud F,
et al: Infectious diseases in the first year of life, perinatal
characteristics and childhood acute leukaemia. Br J Cancer.
90:139–145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudant J, Orsi L, Menegaux F, et al:
Childhood acute leukemia, early common infections and allergy: The
ESCALE Study. Am J Epidemiol. 172:1015–1027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dikalioti SK, Chang ET, Dessypris N, et
al: Allergy-associated symptoms in relation to childhood
non-Hodgkin's as contrasted to Hodgkin's lymphomas: a case-control
study in Greece and meta-analysis. Eur J Cancer. 48:1860–1866.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vajdic CM, Falster MO, de Sanjose S, et
al: Atopic disease and risk of non-Hodgkin lymphoma: an InterLymph
pooled analysis. Cancer Res. 69:6482–6489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang JS, Tsai YW, Tsai CR and Wiemels JL:
Allergy and risk of childhood acute lymphoblastic leukemia: a
population-based and record-based study. Am J Epidemiol.
176:970–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spector L, Groves F, DeStefano F, et al:
Medically recorded allergies and the risk of childhood acute
lymphoblastic leukaemia. Eur J Cancer. 40:579–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hughes AM, Lightfoot T, Simpson J, et al:
Allergy and risk of childhood leukaemia: results from the UKCCS.
Int J Cancer. 121:819–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cooper GS, Kamel F, Sandler DP, Davey FR
and Bloomfield CD: Risk of adult acute leukemia in relation to
prior immune-related conditions. Cancer Epidemiol Biomarkers Prev.
5:867–872. 1996.PubMed/NCBI
|
|
21
|
Soderberg KC, Jonsson F, Winqvist O,
Hagmar L and Feychting M: Autoimmune diseases, asthma and risk of
haematological malignancies: a nationwide case-control study in
Sweden. Eur J Cancer. 42:3028–3033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cozen W, Cerhan JR, Martinez-Maza O, et
al: The effect of atopy, childhood crowding and other
immune-related factors on non-Hodgkin lymphoma risk. Cancer Causes
Control. 18:821–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Linabery AM, Jurek AM, Duval S and Ross
JA: The association between atopy and childhood/adolescent
leukemia: a meta-analysis. Am J Epidemiol. 171:749–764. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smith CT, Williamson PR and Marson AG:
Investigating heterogeneity in an individual patient data
meta-analysis of time to event outcomes. Stat Med. 24:1307–1319.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grulich AE, Vajdic CM, Kaldor JM, et al:
Birth order, atopy and risk of non-Hodgkin lymphoma. J Natl Cancer
Inst. 97:587–594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee WJ, Cantor KP, Berzofsky JA, Zahm SH
and Blair A: Non-Hodgkin's lymphoma among asthmatics exposed to
pesticides. Int J Cancer. 111:298–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee WJ, Purdue MP, Stewart P, et al:
Asthma history, occupational exposure to pesticides and the risk of
non-Hodgkin's lymphoma. Int J Cancer. 118:3174–3176. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rudant J, Orsi L, Monnereau A, et al:
Childhood Hodgkin's lymphoma, non-Hodgkin's lymphoma and factors
related to the immune system: the Escale Study (SFCE). Int J
Cancer. 129:2236–2247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schuz J, Morgan G, Bohler E, Kaatsch P and
Michaelis J: Atopic disease and childhood acute lymphoblastic
leukemia. Int J Cancer. 105:255–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vajdic CM, Fritschi L, Grulich AE, et al:
Atopy, exposure to pesticides and risk of non-Hodgkin lymphoma. Int
J Cancer. 120:2271–2274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen W, Shu XO, Linet MS, et al: Allergic
disorders and the risk of childhood acute lymphoblastic leukemia
(United States). Cancer Causes Control. 11:303–307. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burnet M: Cancer: a biological approach.
III. Viruses associated with neoplastic conditions. IV. Practical
applications. Br Med J. 1:841–847. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H and Diepgen TL: Is atopy a
protective or a risk factor for cancer? A review of epidemiological
studies. Allergy. 60:1098–1111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hellstrand K: Histamine in cancer
immunotherapy: a preclinical background. Semin Oncol. 29:35–40.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gail MH and Benichou J: Encyclopedia of
epidemiologic methods. John Wiley & Sons Ltd.; New York:
2000
|
|
38
|
Infante-Rivard C and Jacques L: Empirical
study of parental recall bias. Am J Epidemiol. 152:480–486. 2000.
View Article : Google Scholar : PubMed/NCBI
|