Introduction
Colorectal cancer (CRC) is the third most common
malignancy worldwide, with 1.2 million new diagnoses and >0.8
million CRC-related deaths worldwide annually. Disease progression
results in metastasis to other organs, such as the liver, and is
associated with higher mortality rates (1). The likelihood of successful complete
excision is therefore significantly increased if surgery is
performed during the early stages. Thus, early detection of CRC is
crucial for maximizing the chances of complete cure (2). The measurement of serum-based tumor
biomarkers is a promising screening method for the detection of
CRC. C-reactive protein (CRP) and carbohydrate antigen 19-9
(CA19-9) are among the most commonly used serum-based
tumor-associated antigens in the management of CRC patients
(3,4).
In this study, we investigated the potential
usefulness of angiopoietin-like protein 2 (ANGPTL2) as a candidate
biomarker for CRC. ANGPTL2 is a secreted protein that regulates
angiogenesis in vivo (5).
Angiogenic factors produced by tumor cells play an important role
in tumor growth. Additionally, this protein is reported to be a
regulatory factor of chronic inflammation (6–10). Endo
et al (9) reported that
ANGPTL2 is a promising biomarker for diagnosing human lung and
breast cancers. Those reports raised the possibility that ANGPTL2
may also be a candidate biomarker for other types of cancer. We
previously demonstrated that ANGPTL2 is upregulated in a gastric
cancer cell line and in gastric cancer patients, demonstrating that
this protein is a clinically useful biomarker for gastric cancer
(11). However, the diagnostic
usefulness of ANGPTL2 in CRC has not yet been investigated. In this
study, we first examined the expression of ANGPTL2 in 7 CRC cell
lines, namely Caco-2, LoVo, WiDr, Colo320, Colo205, CW-2 and
NCC-CoC-K115P. Subsequently, we compared the ANGPTL2 concentrations
in the serum of CRC patients and healthy individuals to evaluate
the sensitivity and specificity of this protein as a predictive
biomarker for CRC.
Materials and methods
Cells
A total of 7 human CRC cell lines, namely Caco-2,
LoVo, WiDr, Colo320, Colo205, CW-2 and NCC-CoC-K115P) were
purchased from the RIKEN BioResource Center (Tsukuba, Ibaraki,
Japan) and the Japanese Collection of Research Bioresources Cell
Bank (Ibaraki, Osaka, Japan). Caco-2 cells were cultured in minimum
essential medium with 0.1 mmol/l non-essential amino acid solution,
20% (w/v) fetal bovine serum (FBS), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in 5% CO2. LoVo cells were
cultured in Ham's F-12 medium with 10% (v/v) FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. WiDr cells were cultured in
Dulbecco's modified Eagle's medium with 5 mmol/l HEPES, 10% (v/v)
FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. NCC-CoC-K115P
cells were cultured in RPMI-1640 medium with 20% (v/v) FBS, 100
U/ml penicillin and 100 µg/ml streptomycin. Colo320, Colo205 and
CW-2 cells were cultured in RPMI-1640 medium with 10% (v/v) FBS,
100 U/ml penicillin and 100 µg/ml streptomycin.
Cell culture
The 7 human CRC cell lines were seeded in 6-well
plates (6×105 cells/well) and incubated at 37°C in 5%
CO2. The media in the wells were changed daily. The
cells were collected from the wells every day by trypsinization and
the cell number was determined using a hemocytometer. Prior to the
analysis, samples of medium from cultured cells were stored at
−80°C.
Serum samples
Serum samples were obtained from 56 participants who
attended the clinic between May, 2013 and February, 2014 at the
Nanpuh Hospital (Kagoshima, Japan). The participants included 15
patients with CRC [mean age, 63.8 years, standard deviation (SD),
9.4 years] and 41 healthy controls with normal mucosa (mean age,
47.7 years; SD, 9.7 years). Of the 15 CRC patients, 14 were
diagnosed with adenocarcinoma and 1 with mucinous adenocarcinoma.
The patient group included 6 patients diagnosed with clinical stage
0-I, 6 patients with clinical stage II and 3 patients with clinical
stage III disease. Cancer staging was based on a routine
histopathological analysis and clinical assessment, according to
the tumor-node-metastasis classification. Tumors were classified
according to the recommendations of the 5th International Union
Against Cancer. The characteristics of the subjects are summarized
in Table I. Informed consent was
obtained from all the participants. The study design was approved
by the Ethics Committee of Nanpuh Hospital, Kagoshima Kyosaikai,
Public Interest Inc. Association, Japan. Clinical examinations were
performed according to the principles of the Declaration of
Helsinki.
 | Table I.Characteristics of the subjects. |
Table I.
Characteristics of the subjects.
| Characteristics | CRC patients
(n=15) | Healthy controls
(n=41) | Total (n=56) |
|---|
| Age, years |
|
|
|
| Mean ±
SD | 63.8±9.4 | 47.7±9.7 | 52.0±11.9 |
|
Range | 44–80 | 35–75 | 35–80 |
| Gender |
|
|
|
| Male | 13 | 22 | 35 |
|
Female | 2 | 19 | 21 |
| BMI
(kg/m2) |
|
|
|
| Mean ±
SD | 23.2±3.4 | 22.1±2.7 | 22.4±2.9 |
|
Range | 16.8–29.0 | 17.4–29.5 | 16.8–29.5 |
| Tumor stage |
|
|
|
| 0-I | 6 | – | 6 |
| II | 6 | – | 6 |
| III | 3 | – | 3 |
| Depth of
invasion |
|
|
|
| M | 3 | – | 3 |
| SM | 0 | – | 0 |
| MP | 3 | – | 3 |
| SS/A | 3/1 | – | 3/1 |
|
SE/SI | 4/1 | – | 4/1 |
| Degree of
differentiation |
|
|
|
| Moderate
to poor | 2 | – | 2 |
| High to
moderate | 5 | – | 5 |
| High | 8 | – | 8 |
Measurement of biomarkers in cell
culture media and serum
The concentrations of ANGPTL2 in human serum and
cell culture medium samples were determined using an ANGPTL2
enzyme-linked immunosorbent assay kit (Immuno-Biological
Laboratories, Co., Ltd., Gunma, Japan). The concentrations of CRP
in the serum were determined by latex agglutination using BM6050
(Kyowa Medex, Co., Ltd., Tokyo, Japan) in accordance with the
manufacturer's instructions. The concentrations of CA19-9 in the
serum were determined using the electrochemiluminescence
immunoassay with LUMIPULSE G1200® (Fujirebio, Co., Ltd., Tokyo,
Japan) in accordance with the manufacturer's instructions.
Statistical analysis
The correlation of the serum ANGPTL2 concentration
with the patients' age and tumor size was analyzed using Pearson's
correlation analysis. The correlation of the serum ANGPTL2
concentration with the degree of differentiation and depth of tumor
invasion were analyzed using Spearman's rank correlation analysis.
The statistical difference between ANGPTL2 concentration in the
serum of CRC patients and healthy individuals was analyzed using
the rank, non-parametric, statistical Mann-Whitney U test. Data are
presented as means ± SD. A receiver operating characteristic (ROC)
curve was constructed to evaluate the diagnostic performance of
ANGPTL2 concentration in differentiating between CRC patients and
healthy individuals. P<0.05 was considered to indicate a
statistically significant difference.
Results
ANGPTL2 expression in CRC cell
lines
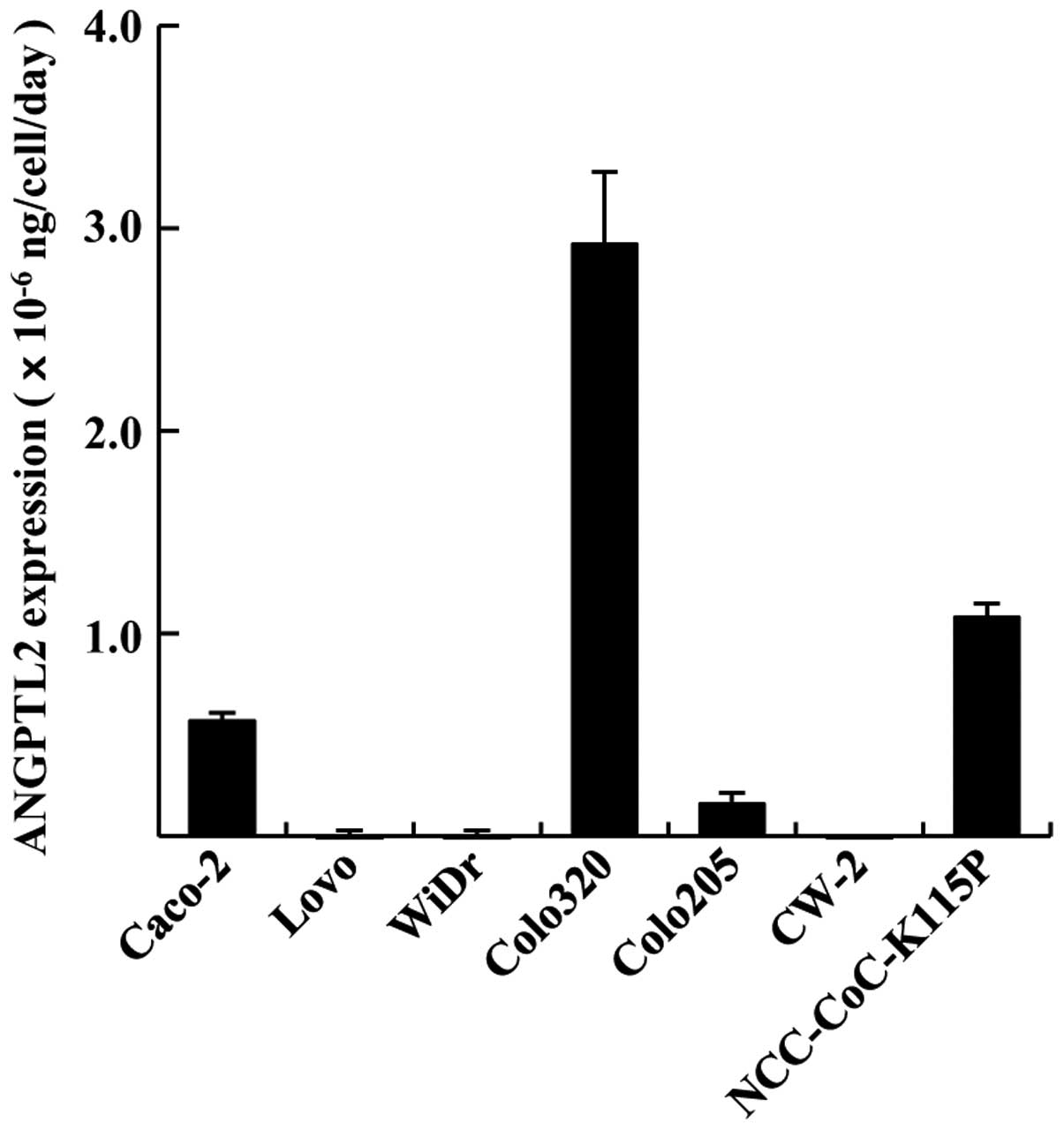
We first investigated ANGPTL2 expression in 7 CRC
cell lines. The ANGPTL2 expression rates of the cell lines at the
second day of cell culture are presented in Fig. 1. The expression rate in Caco-2,
Colo320, Colo205 and NCC-CoC-K115P cells was
0.57±0.04×10−6, 2.92±0.36×10−6,
0.16±0.06×10−6 and 1.08±0.07×10−6
ng/cell/day. LoVo, WiDr and CW-2 cell lines exhibited very low
expression of ANGPTL2. Thus, Caco-2, Colo320, Colo205 and
NCC-CoC-K115P cells exhibited comparatively high ANGPTL2
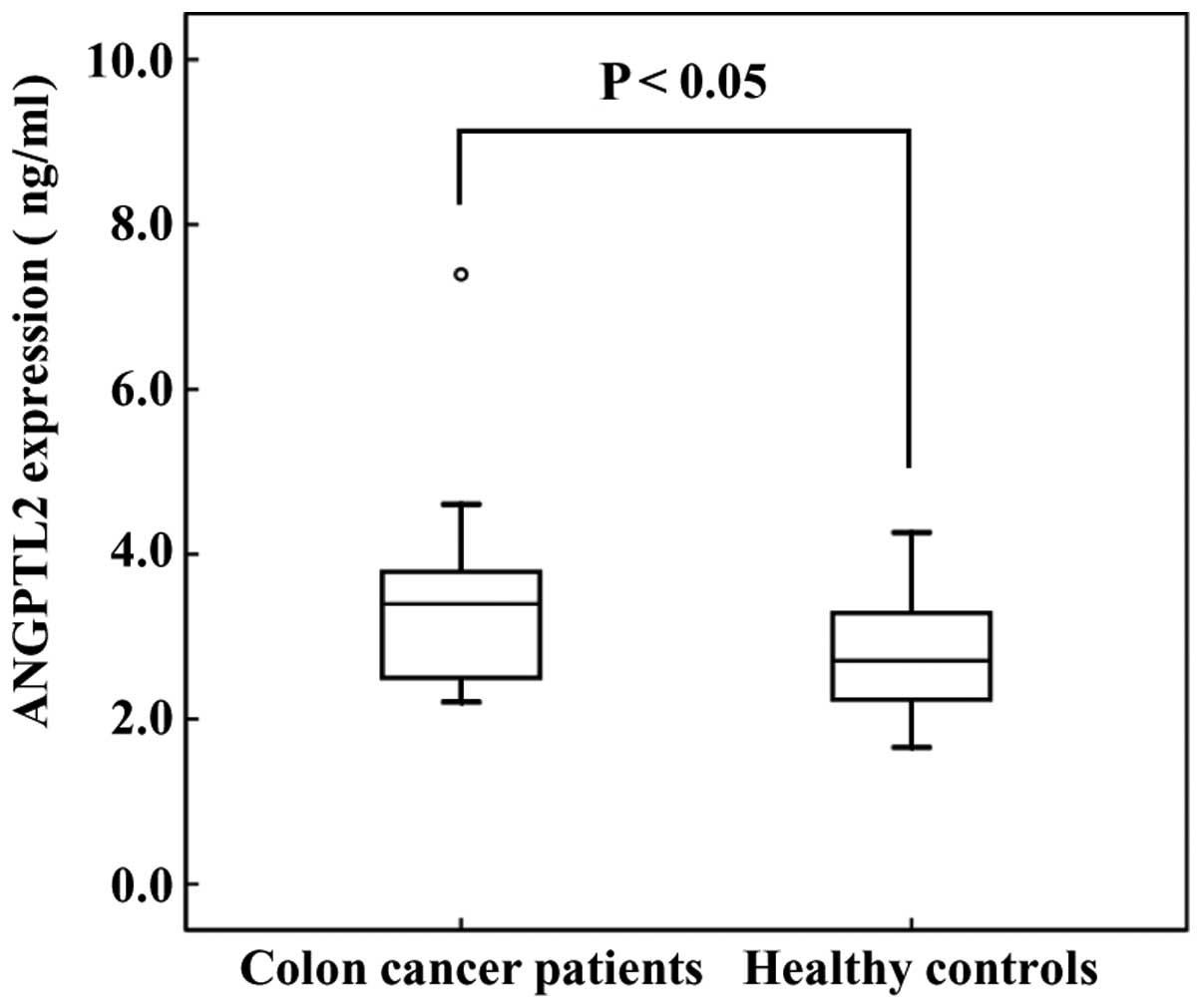
production. Subsequently, we evaluated ANGTPL2 levels in the serum
of CRC patients. The serum levels of ANGPTL2 in CRC patients were
higher compared with those in healthy controls (3.45±1.30 vs.
2.74±0.64 ng/ml, respectively; Fig.
2).
Correlation between serum ANGPTL2
concentration and different variables in CRC patients
There was no correlation between the ANGPTL2 level
and patient age (r=-0.034, P=0.903), tumor size (r=-0.029,
P=0.924), degree of tumor differentiation (r=0.069, P=0.806), or
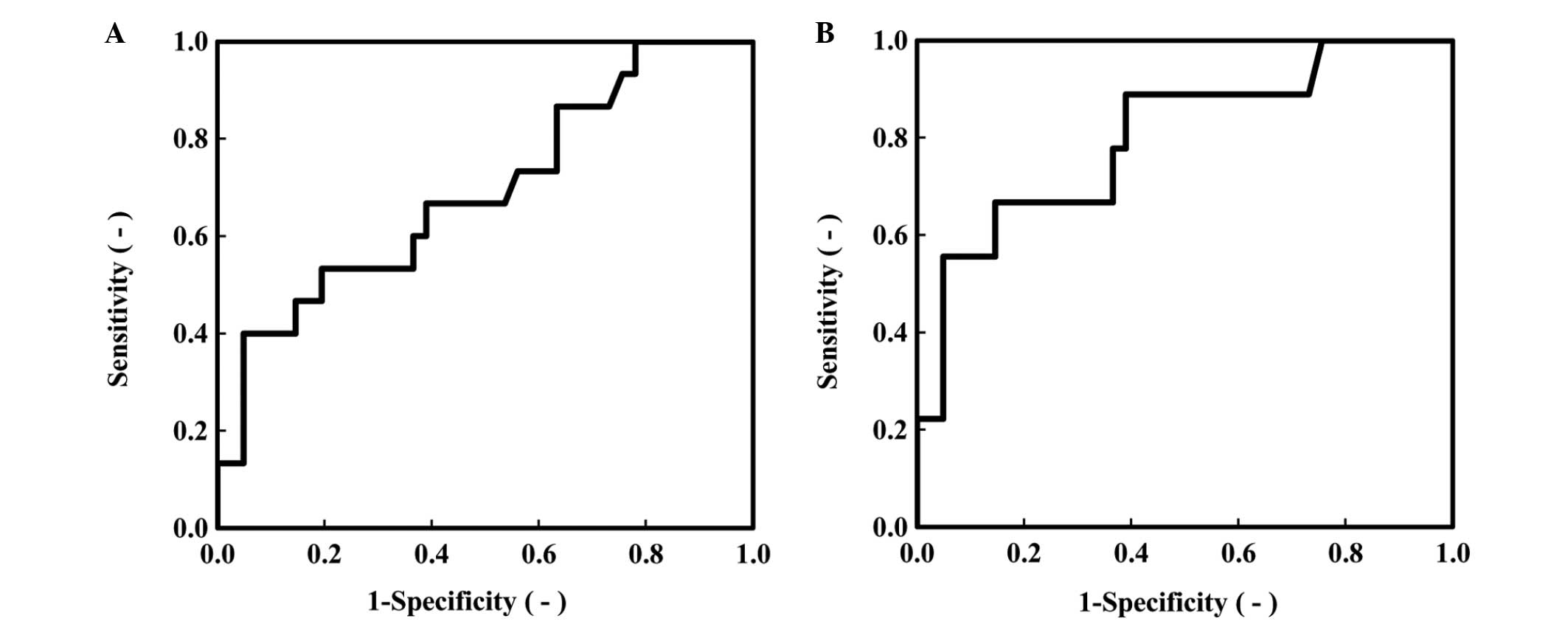
depth of tumor invasion (r=0.327, P=0.234) (Table II). We further evaluated the
potential of ANGPTL2 as a biomarker of CRC by employing an ROC
analysis (Fig. 3, Table III). The area under the ROC curve
(AUC) for ANGPTL2 was 0.691 [P=0.030, 95% confidence interval (CI):
0.529–0.853], the AUC for CRP was 0.763 (P=0.003, 95% CI:
0.591–0.934) and the AUC for CA19-9 was 0.630 (P=0.139, 95% CI:
0.463–0.797). Additionally, we performed the same ROC analysis in
CRC patients with stage II and III tumors (Fig. 3B, Table
III). The AUC for ANGPTL2 was 0.801 (P=0.005, 95% CI:
0.632–0.970), the AUC for CRP was 0.911 (P<0.005, 95% CI:
0.784–1.037) and the AUC for CA19-9 was 0.598 (P=0.363, 95% CI:
0.390–0.805) (Table III). Tumor
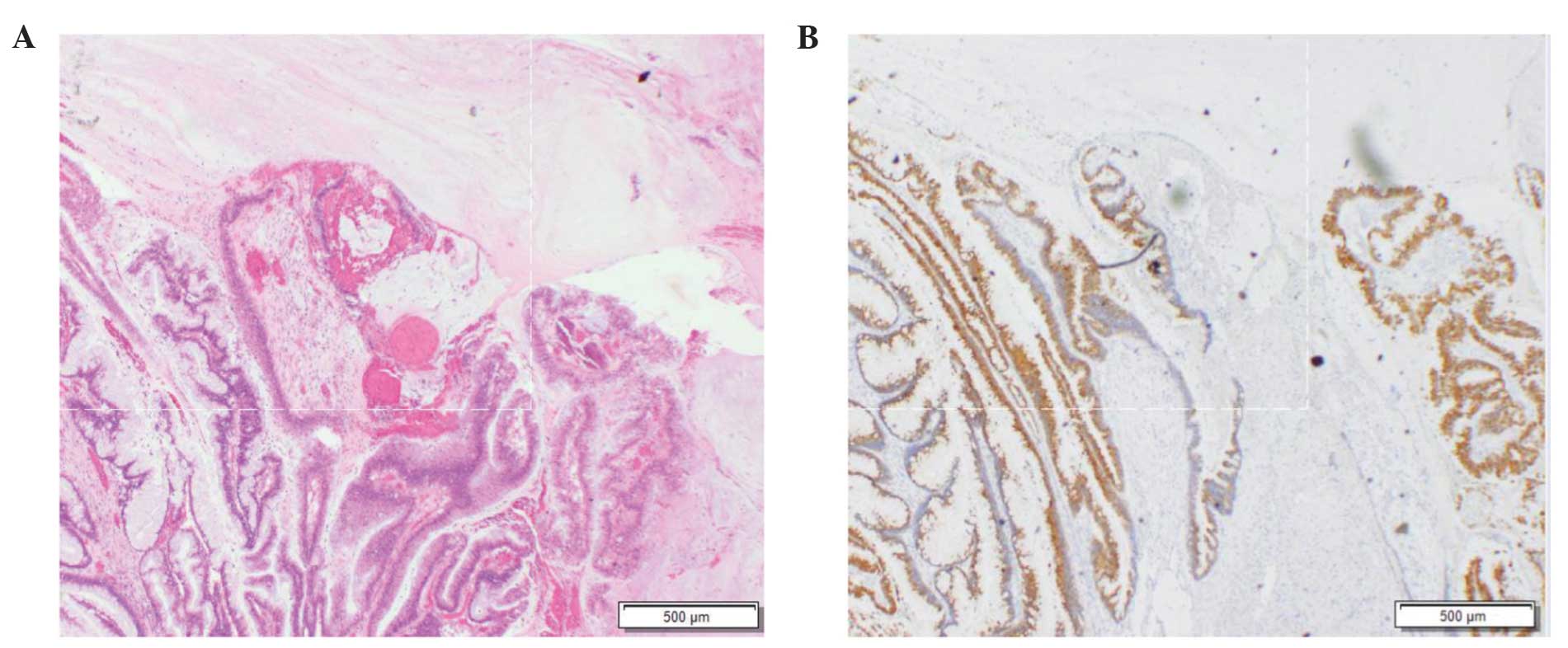
samples from CRC patients who exhibited the highest ANTPTL2 level
were positive for mucin 2 and mucin 5AC (Fig. 4).
 | Table II.Correlation between serum ANGPTL2
concentration and different variables in CRC patients (n=15). |
Table II.
Correlation between serum ANGPTL2
concentration and different variables in CRC patients (n=15).
| Variables | Correlation
coefficient | P-value |
|---|
| Age, years | −0.034 | 0.903 |
| Tumor size | −0.029 | 0.924 |
| Degree of
differentiation | 0.069 | 0.806 |
| Depth of
invasion | 0.327 | 0.234 |
 | Table III.Summary of ROC curve analysis for
ANGPTL2, CRP and CA19-9. |
Table III.
Summary of ROC curve analysis for
ANGPTL2, CRP and CA19-9.
| Biomarkers | AUC | SE | P-value | 95% CI |
|---|
| Total data (stage
0-III) |
|
|
|
|
|
ANGPTL2 | 0.691 | 0.083 | 0.030a | 0.529–0.853 |
| CRP | 0.763 | 0.087 | 0.003a | 0.591–0.934 |
|
CA19-9 | 0.630 | 0.085 | 0.139 | 0.463–0.797 |
| Stage ≥II |
|
|
|
|
|
ANGPTL2 | 0.801 | 0.086 | 0.005b | 0.632–0.970 |
| CRP | 0.911 | 0.064 |
<0.005b | 0.784–1.037 |
|
CA19-9 | 0.598 | 0.106 | 0.363 | 0.390–0.805 |
Discussion
To the best of our knowledge, this is the first
reported investigation of ANGPTL2 expression in CRC. The Colo320
cell line exhibited the highest level of ANGPTL2 expression. This
cell line represents a migratory phenotype with extensive
epithelial-to-mesenchymal transition (EMT) and scored the highest
in terms of signatures associated with worse overall survival and
higher risk of recurrence (12). In
our previous study, we reported that the HGC-27 gastric cancer cell
line also exhibited a high level of ANGPTL2 expression; this cell
line exhibits a low expression level of E-cadherin (13). These results indicate that the
expression of ANGPTL2 may be associated with EMT and E-cadherin.
However, further studies are required to elucidate the mechanism
underlying the enhanced expression of ANGPTL2.
Additionally, we demonstrated that the serum ANGTPL2
levels in CRC patients were higher compared with those in healthy
controls. Furthermore, the ROC analysis demonstrated that the
diagnostic ability of ANGPTL2 was marginally lower compared with
that of the classic biomarker CRP and higher compared with that of
CA19-9. These results suggest that simultaneous measurement of
ANGPTL2 and the classic biomarkers may reduce the likelihood of
overlooking CRC.
It has been reported that colorectal mucinous
adenocarcinoma is associated with a poor prognosis. Mucinous adeno
carcinoma is often detected at an advanced stage (14). In this study, a CRC patient with
mucinous mucinous adenocarcinoma carcinoma exhibited the highest
ANGPTL2 level, suggesting that high ANGPTL2 expression may be a
predictive biomarker for that type of cancer.
Acknowledgements
This study was supported in part by the Division of
Gene Research, Kagoshima University, the Division of
Gastrointestinal Surgery, Nanpuh Hospital, the Division of
Diagnostic Pathology, Nanpuh Hospital and the Division of Clinical
Laboratory, Nanpuh Hospital.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshida N, Yagi N, Naito Y and Yoshikawa
T: Safe procedure in endoscopic submucosal dissection for
colorectal tumors focused on preventing complications. World J
Gastroenterol. 16:1688–1695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chapman MA, Buckley D, Henson DB and
Armitage NC: Preoperative carcinoembryonic antigen is related to
tumour stage and long-term survival in colorectal cancer. Br J
Cancer. 78:1346–1349. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuusela P, Jalanko H, Roberts P, Sipponen
P, Mecklin JP, Pitkänen R and Mäkelä O: Comparison of CA 19-9 and
carcinoembryonic antigen (CEA) levels in the serum of patients with
colorectal diseases. Br J Cancer. 49:135–139. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hato T, Tabata M and Oike Y: The role of
angiopoietin-like proteins in angiogenesis and metabolism. Trends
Cardiovasc Med. 18:6–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabata M, Kadomatsu T, Fukuhara S, et al:
Angiopoietin-like protein 2 promotes chronic adipose tissue
inflammation and obesity-related systemic insulin resistance. Cell
Metab. 10:178–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kadomatsu T, Tabata M and Oike Y:
Angiopoietin-like proteins: Emerging targets for treatment of
obesity and related metabolic diseases. FEBS J. 278:559–564. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aoi J, Endo M, Kadomatsu T, et al:
Angiopoietin-like protein 2 is an important facilitator of
inflammatory carcinogenesis and metastasis. Cancer Res.
71:7502–7512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Endo M, Nakano M, Kadomatsu T, et al:
Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical
driver of metastasis. Cancer Res. 72:1784–1794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okada T, Tsukano H, Endo M, et al:
Synoviocyte-derived angiopoietin-like protein 2 contributes to
synovial chronic inflammation in rheumatoid arthritis. Am J Pathol.
176:2309–2319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshinaga T, Shigemitsu T, Nishimata H,
Takei T and Yoshida M: Angiopoietin-like protein 2 is a potential
biomarker for gastric cancer. Mol Med Rep. 11:2653–2658.
2015.PubMed/NCBI
|
|
12
|
Christensen J, El-Gebali S, Natoli M, et
al: Defining new criteria for selection of cell-based intestinal
models using publicly available databases. BMC Genomics.
13:2742012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuwabara Y, Yamada T, Yamazaki K, Du WL,
Banno K, Aoki D and Sakamoto M: Establishment of an ovarian
metastasis model and possible involvement of E-cadherin
down-regulation in the metastasis. Cancer Sci. 99:1933–1939.
2008.PubMed/NCBI
|
|
14
|
Symonds DA and Vickery AL: Mucinous
carcinoma of the colon and rectum. Cancer. 37:1891–1900. 1976.
View Article : Google Scholar : PubMed/NCBI
|