Introduction
Renal cell carcinoma (RCC) represents 2.4% of all
malignancies worldwide (http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx).
In addition, RCC accounts for 90% of renal tumors, with 85% of RCCs
being of the clear cell type (1).
According to the Surveillance of Epidemiology and End Results data
from the USA, the 5-year survival rate of advanced renal cancer is
~12.6% (http://seer.cancer.gov/statfacts/html/kidrp.html).
Cytoreduction surgery followed by systemic therapy is generally
recommended for patients with advanced disease (2,3). Tyrosine
kinase inhibitors are widely used as first- and second-line
treatment and, to date, 7 such agents, including sorafenib, have
been approved by the Food and Drug Administration for the treatment
of advanced RCC [National Comprehensive Cancer Network (NCCN)
guidelines 2014, www.nccn.com].
Sorafenib tosylate is a small molecule that inhibits
multiple serine/threonine kinases, RAF and other receptor kinases,
e.g., vascular endothelial growth factor receptor (VEGFR)-1, -2 and
-3, platelet-derived growth factor receptor-β and other receptors
(4–8).
While this drug was considered as a standard of care for patients
who progressed on prior cytokine therapy (9–11) or
following treatment with a tyrosine kinase inhibitor (12,13), only
one randomized phase II clinical study (14) has been published on using sorafenib as
first-line therapy.
Thus, the aim of this multicenter study was to
confirm the efficacy and evaluate the safety of sorafenib as
first-line therapy in patients with advanced or metastatic RCC.
Patients and methods
Patient selection
A total of 75 eligible patients from 8 centers in 3
Middle East countries (Egypt, Lebanon and Saudi Arabia) were
recruited.
The eligibility criteria included pathologically
proven advanced RCC, Eastern Cooperative Oncology Group performance
status 0–2 and measurable disease. The patients were administered
sorafenib as first-line therapy, as they were considered ineligible
to receive other approved first-line therapies and, in the
investigator's opinion, they were reasonably likely to benefit from
single-agent sorafenib treatment.
Adequate renal, hepatic and bone marrow function was
required, as defined by a serum creatinine ≤2.0 × institutional
upper limit of normal (ULN), total bilirubin ≤1.5 × ULN, aspartate
aminotransferase/alanine aminotransferase ≤2.5 × ULN, leukocyte
count ≥3,000/µl, absolute neutrophil count ≥1,500/µl and platelet
count ≥75,000/µl. The patients provided written informed consent
prior to receiving sorafenib.
The exclusion criteria included serious
cardiovascular disease, a life expectancy of <2 months,
metastatic brain or meningeal tumors, recent or active bleeding
diathesis and any prior systemic therapy for RCC.
The study protocol was approved by the review boards
of all the participating institutions. All the procedures were
conducted according to the principles of the Declaration of
Helsinki.
Baseline evaluation
The baseline patient evaluation included medical
history and physical examination, laboratory investigations
(complete blood count and blood chemistry) and tumor radiological
imaging, including computed tomography (CT) scan or magnetic
resonance imaging (MRI) of the chest, abdomen and pelvis, isotope
bone scan and brain MRI or CT scan.
Treatment protocol
The treatment was administered as 400 mg sorafenib
orally, twice daily, continuously in 4-week cycles. Dose increases
were not permitted, while dose reductions to 400 mg daily and then
every other day was allowed, depending on the type and severity of
toxicity encountered and provided that the criteria for patient
withdrawal from the study treatment were not met. The patients
continued to receive treatment with sorafenib until development of
intolerable toxicity, disease progression, or withdrawal of consent
for any reason.
Response to treatment and adverse
events
Tumor assessments were performed according to the
Response Evaluation Criteria in Solid Tumors at baseline and every
8 weeks thereafter, or earlier if tumor progression was clinically
suspected (15). Any adverse events
were reported and graded according to the Common Toxicity Criteria,
version 4.0 [National Cancer Institute. Common Terminology Criteria
for Adverse Events (CTCAE) Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf].
All the responses were reviewed by independent experts at the study
completion, including simultaneous review of patient files and
radiological images.
Statistical analysis
Patient demographic data and baseline
characteristics were analyzed by summary statistics for
quantitative variables and frequency tables for qualitative
variables. Efficacy data were summarized by frequency tables.
Overall survival was defined as the time from randomization to
death from any cause; progression-free survival was calculated from
the date of first administration of sorafenib to the investigator's
assessment or radiological documentation of disease progression.
Survival was analyzed by the Kaplan-Meier estimate. Statistical
analysis was performed using IBM SPSS software, version 20 (IBM
SPSS, Chicago, IL, USA).
Results
Participating institutions
Between October, 2008 and February, 2011, 75
eligible patients were recruited to participate in this study. Of
the 75 patients, 65 were from Egypt, 8 from Lebanon and 2 from
Saudi Arabia. The majority of the Egyptian patients were recruited
from the National Cancer Institute, Cairo University (n=43), while
the remaining 22 patients were recruited from four additional
centers (two private centers, the Clinical Oncology Department of
Alexandria University and the Maadi Military Hospital). Two centers
in Lebanon participated in the study (American University in
Beirut, 3 patients; and Hôtel-Dieu de France, 5 patients). The
remaining 2 patients were recruited from the National Guard
Hospital in Saudi Arabia.
Patient characteristics
The patients comprised 48 men and 27 women, with a
median age of 52 years (range, 19–78 years). A total of 50 patients
had clear cell carcinoma, 17 had papillary carcinoma and 8 had
other pathological subtypes (5 chromophobe, 1 medullary and 2
unclassified RCCs). The majority of the patients (n=55; 73%) had
undergone prior nephrectomy.
A total of 67 patients presented with metastatic
disease, while 8 patients presented with regional residual lesions
or local recurrence. Multiple organ metastases (lung, liver, bone
and lymph nodes) were present in 46 patients, while only lung,
liver, or lymph node metastases were found in 15, 3 and 3 patients,
respectively (Table I).
 | Table I.Lesions of the 75 patients at
presentation. |
Table I.
Lesions of the 75 patients at
presentation.
| Lesions | Randomized patients
(n=75) | Evaluable patients
(n=60) |
|---|
| Residual mass | 4 | 3 |
| Local recurrence | 4 | 3 |
| Metastatic lung
lesions only | 15 | 13 |
| Metastatic liver
lesions only | 3 | 2 |
| Metastatic lymph node
only | 3 | 2 |
| Multiple organ
metastasis (lung, liver, bone, lymph nodes) | 46 | 37 |
Adverse events
The duration of sorafenib treatment was 1–137 weeks,
with a median duration of 21 weeks. The most commonly reported
grade 3/4 adverse events were hand-foot syndrome, fatigue,
diarrhea, elevated liver enzymes, vomiting and generalized pain.
Anemia and hypertension occurred with a lower frequency (Table II). Certain adverse events were
severe enough to require hospitalization (11 patients).
 | Table II.AEs according to the CommonToxicity
Criteria v.4.0. |
Table II.
AEs according to the CommonToxicity
Criteria v.4.0.
|
| All patients | Evaluable |
|---|
|
|
|
|
|---|
| Symptoms | Grade 3 | Grade 4 | Grade 3 | Grade 4 |
|---|
| Pain | 2 | 0 | 2 | 0 |
| Fatigue | 2 | 1 | 0 | 1 |
| Hand-foot
syndrome | 16 | 1 | 16 | 1 |
| Elevated liver
enzymes | 3 | 0 | 2 | 0 |
| Vomiting | 2 | 0 | 1 | 0 |
| Diarrhea | 3 | 0 | 3 | 0 |
| Anemia | 1 | 0 | 1 | 0 |
| Hypertension | 1 | 0 | 1 | 0 |
Response
Of the 75 patients included in the study, 15 were
non-evaluable for treatment response due to the patient's wish to
discontinue treatment (n=5), death prior to evaluation (n=4),
serious adverse event and/or drug toxicity (n=3), refusal to
initiate treatment following enrollment (n=2) and non-compliance
(n=1).
During a median observation time of 53.5 weeks
(range, 8.5–192 weeks), 1 patient achieved a complete response and
6 patients had partial remissions, with an overall response rate of
11.7% (7/60 patients). The duration of the complete remission was
91 weeks, while the partial remission durations ranged between 9
and 35 weeks, with a median of 23 weeks. Additionally, 37 patients
(61.7%) exhibited disease stabilization. Thus, disease control was
achieved in 44/60 patients (73%). The disease control rate was not
affected by pathological subtype (clear cell vs. non-clear cell),
being 68.4% (26/38 patients) and 81.8% (18/22 patients),
respectively (P=0.52).
At the time of study completion, 6 patients remained
alive. In an intention-to-treat analysis, the median
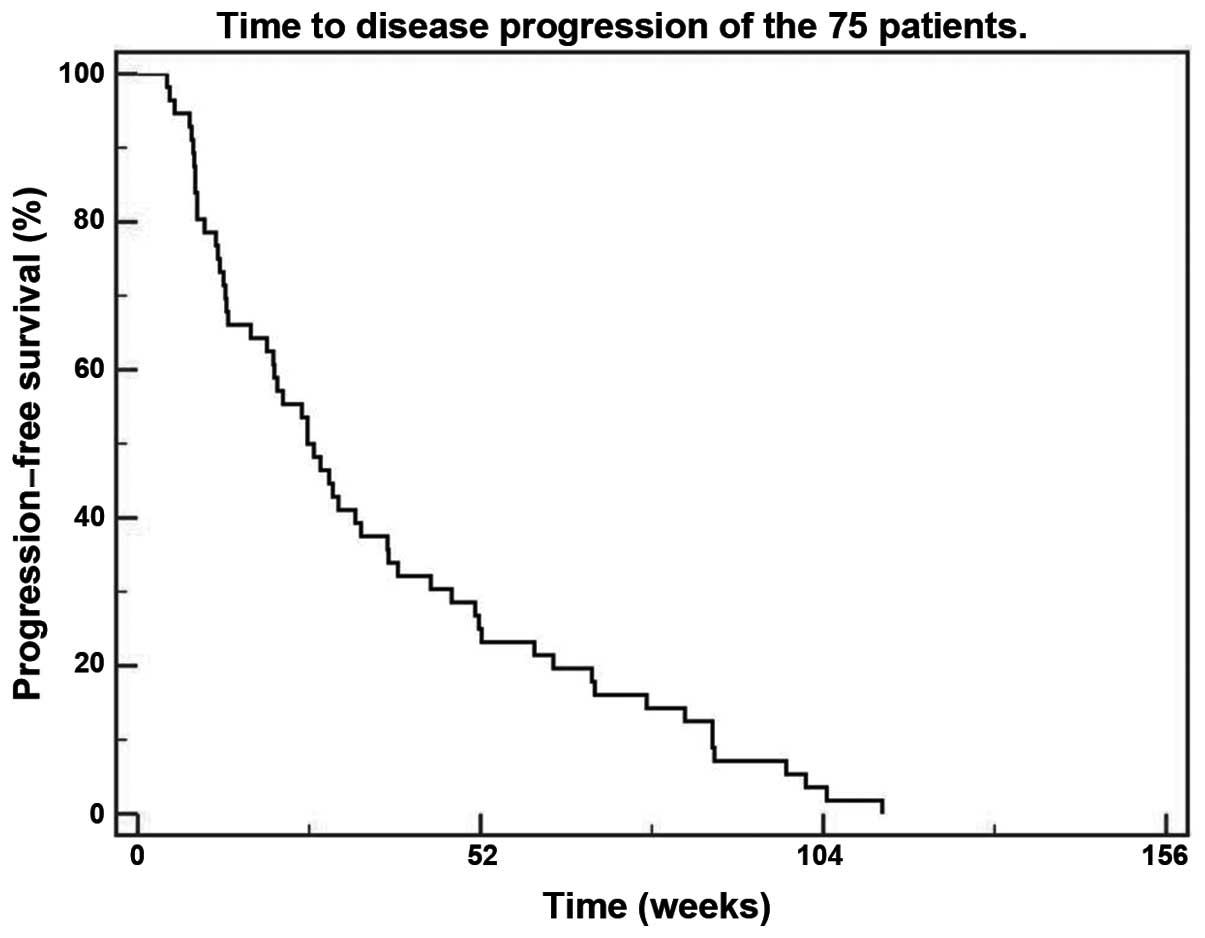
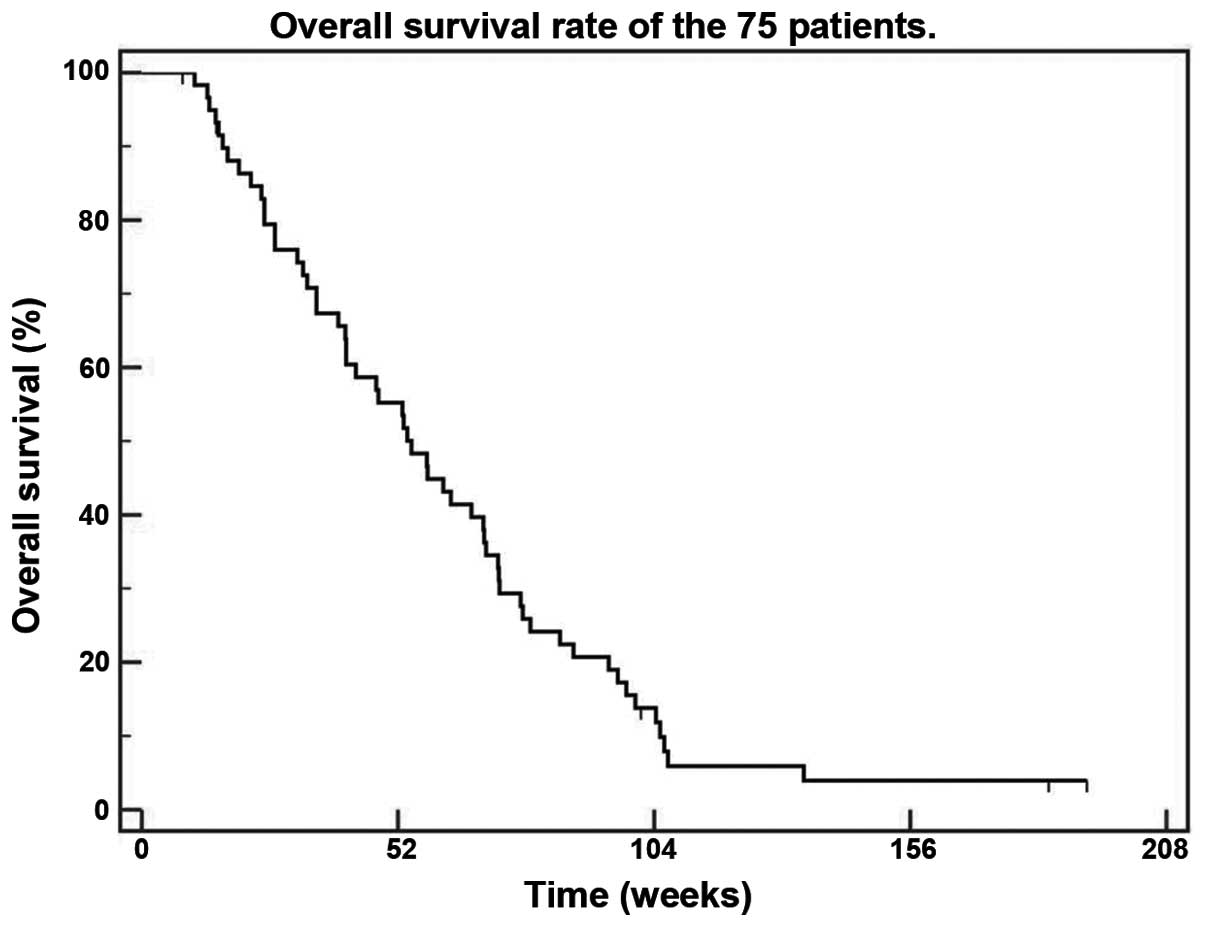
time-to-disease progression (TDP) was 25.7 weeks (Fig. 1), while the median overall survival
was 54.8 weeks (Fig. 2). The median
TDP and the median overall survival were not affected by the
pathological subtype, being 25.7 and 53.1 weeks for the clear cell
type (P=0.1) and 38 and 66.9 weeks for the non-clear cell subtypes
(P=0.8), respectively.
Discussion
The systemic treatment options for advanced RCC
were, until recently, limited to cytokine therapy, e.g., interferon
and interleukin-2. Targeted therapies, mainly with tyrosine kinase
inhibitors, were introduced over the last few years. These include
agents such as sunitinib, sorafenib, axitinib, temsirolimus,
pazopanib, everolimus and bevacizumab. Tumor histology and risk
factor evaluation are crucial for selecting the targeted agent
suitable for each patient.
Interim data from the phase III TARGET trial on
advanced RCC demonstrated a significant increase, with near
doubling of the progression-free survival for patients receiving
sorafenib vs. placebo (5.5 vs. 2.8 months, respectively). That
trial included >900 patients who exhibited disease progression
following prior therapy (9). This
encouraged investigators to evaluate the effects of sorafenib as
first-line treatment for patients with advanced RCC (14,16,17).
The present study demonstrated that sorafenib,
administered as first-line treatment for patients with advanced
RCC, is tolerable and effective. A previous randomized study by
Escudier et al (14) provided
the first evidence that sorafenib may be administered as front-line
therapy, with a tumor shrinkage rate of 68.2% and a median
progression-free survival of 5.7 months. These figures are
comparable to those of the present study, leading NCCN to list
sorafenib as a category 2A option for the first-line therapy of
advanced RCC (NCCN guidelines 2014, www.nccn.com).
The progression-free survival rate with other targeted agents, such
as sunitinib, pazopanib and temsirolimus, in the front-line setting
was reportedly 11.9, 2 and 5.5 months, respectively, with an
overall objective response rate of ~30% (NCCN guidelines 2014,
www.nccn.com).
The observed clinical activity of the sequential use
of VEGF inhibitors indicates persistent RCC tumor reliance on VEGF
signaling following exposure to different VEGF-binding or other
VEGFR inhibitors. Therefore, response or lack of response to prior
VEGF-targeted therapy does not appear to affect further therapy
with agents exhibiting similar mechanisms of action. Iacovelli
et al (18) recently reported
data from a cohort of patients who had received three lines of
therapy, suggesting that the sequence of VEGF inhibitor followed by
another VEGF inhibitor followed by a mechanistic target of
rapamycin (mTOR) inhibitor may be associated with better survival
compared with a sequence in which an mTOR inhibitor was sandwiched
between two VEGF inhibitors. Phase III trials are required to
further support this concept and may help define an optimal
sequence on an individual patient basis.
There were certain limitations to the present study.
First, this was a single-agent, non-randomized study. Therefore,
the relative benefit of sorafenib as compared with other agents was
not clearly determined. Second, there are certain inherent
limitations to response evaluation criteria, particularly
radiological evaluation, with targeted agents in RCC, including
treatment with sorafenib.
In conclusion, the present study demonstrated that
the use of first-line sorafenib for patients with advanced RCC is a
tolerable and effective treatment option.
References
|
1
|
Karumanchi SA, Merchan J and Sukhatme VP:
Renal cancer: Molecular mechanisms and newer therapeutic options.
Curr Opin Nephrol Hypertens. 11:37–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Flanigan RC, Salmon SE, Blumenstein BA,
Bearman SI, Roy V, McGrath PC, Caton JR Jr, Munshi N and Crawford
ED: Nephrectomy followed by interferon alfa-2b compared with
interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J
Med. 345:1655–1659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choueiri TK, Xie W, Kollmannsberger C,
North S, Knox JJ, Lampard JG, McDermott DF, Rini BI and Heng DY:
The impact of cytoreductive nephrectomy on survival of patients
with metastatic renal cell carcinoma receiving vascular endothelial
growth factor targeted therapy. J Urol. 185:60–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Awada A, Hendlisz A, Gil T, et al: Phase I
safety and pharmacokinetics of BAY 43-9006 administered for 21 days
on/7 days off in patients with advanced, refractory solid tumours.
Br J Cancer. 92:1855–1861. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clark JW, Eder JP, Ryan D, Lathia C and
Lenz HJ: Safety and pharmacokinetics of the dual action Raf kinase
and vascular endothelial growth factor receptor inhibitor, BAY
43-9006, in patients with advanced, refractory solid tumors. Clin
Cancer Res. 11:5472–5480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moore M, Hirte HW, Siu L, Oza A, Hotte SJ,
Petrenciuc O, Cihon F, Lathia C and Schwartz B: Phase I study to
determine the safety and pharmacokinetics of the novel Raf kinase
and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days
off in patients with advanced, refractory solid tumors. Ann Onco.
16:1688–1694. 2005. View Article : Google Scholar
|
|
7
|
Strumberg D, Richly H, Hilger RA, et al:
Phase I clinical and pharmacokinetic study of the Novel Raf kinase
and vascular endothelial growth factor receptor inhibitor BAY
43-9006 in patients with advanced refractory solid tumors. J Clin
Oncol. 23:965–972. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Escudier B, Eisen T, Stadler WM, et al:
TARGET Study Group: Sorafenib in advanced clear-cell renal-cell
carcinoma. N Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eisen T, Bukowski RM, Staehler M, Szczylik
C, Oudard S, Stadler WM, Schwartz B, Simantov R, Shan M and
Escudier B: The Sorafenib TARGETs Clinical Trial Group: Randomized
phase III trial of sorafenib in advanced renal cell carcinoma
(RCC): Impact of crossover on survival. 2006 ASCO Annual Meeting
Proceedings. J Clin Oncol. 24(Suppl 18): 45242006.
|
|
11
|
Bukowski RM, Eisen T, Szczylik C, Stadler
WM, Simantov R, Shan M, Elting J, Pena C and Escudier B: Final
results of the randomized phase III trial of sorafenib in advanced
renal cell carcinoma: Survival and biomarker analysis. ASCO Annual
Meeting Proceedings (Post-Meeting Edition). J Clin Oncol. 25(Suppl
18): 50232007.
|
|
12
|
Di Lorenzo G, Cartenì G, Autorino R, et
al: Phase II study of sorafenib in patients with
sunitinib-refractory metastatic renal cell cancer. J Clin Oncol.
27:4469–4474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garcia JA, Hutson TE, Elson P, Cowey CL,
Gilligan T, Nemec C, Dreicer R, Bukowski RM and Rini BI: Sorafenib
in patients with metastatic renal cell carcinoma refractory to
either sunitinib or bevacizumab. Cancer. 116:5383–5390. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Escudier B, Szczylik C, Hutson TE, et al:
Randomized phase II trial of first-line treatment with sorafenib
versus interferon alfa-2a in patients with metastatic renal cell
carcinoma. J Clin Oncol. 27:1280–1289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park SJ, Lee JL, Park I, et al:
Comparative efficacy of sunitinib versus sorafenib as first-line
treatment for patients with metastatic renal cell carcinoma.
Chemotherapy. 58:468–474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Procopio G, Derosa L, Gernone A, et al:
Sorafenib as first- or second-line therapy in patients with
metastatic renal cell carcinoma in a community setting. Future
Oncol. 10:1741–1750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iacovelli R, Cartenì G, Sternberg CN, et
al: Clinical outcomes in patients receiving three lines of targeted
therapy for metastatic renal cell carcinoma: Results from a large
patient cohort. Eur J Cancer. 49:2134–2142. 2013. View Article : Google Scholar : PubMed/NCBI
|