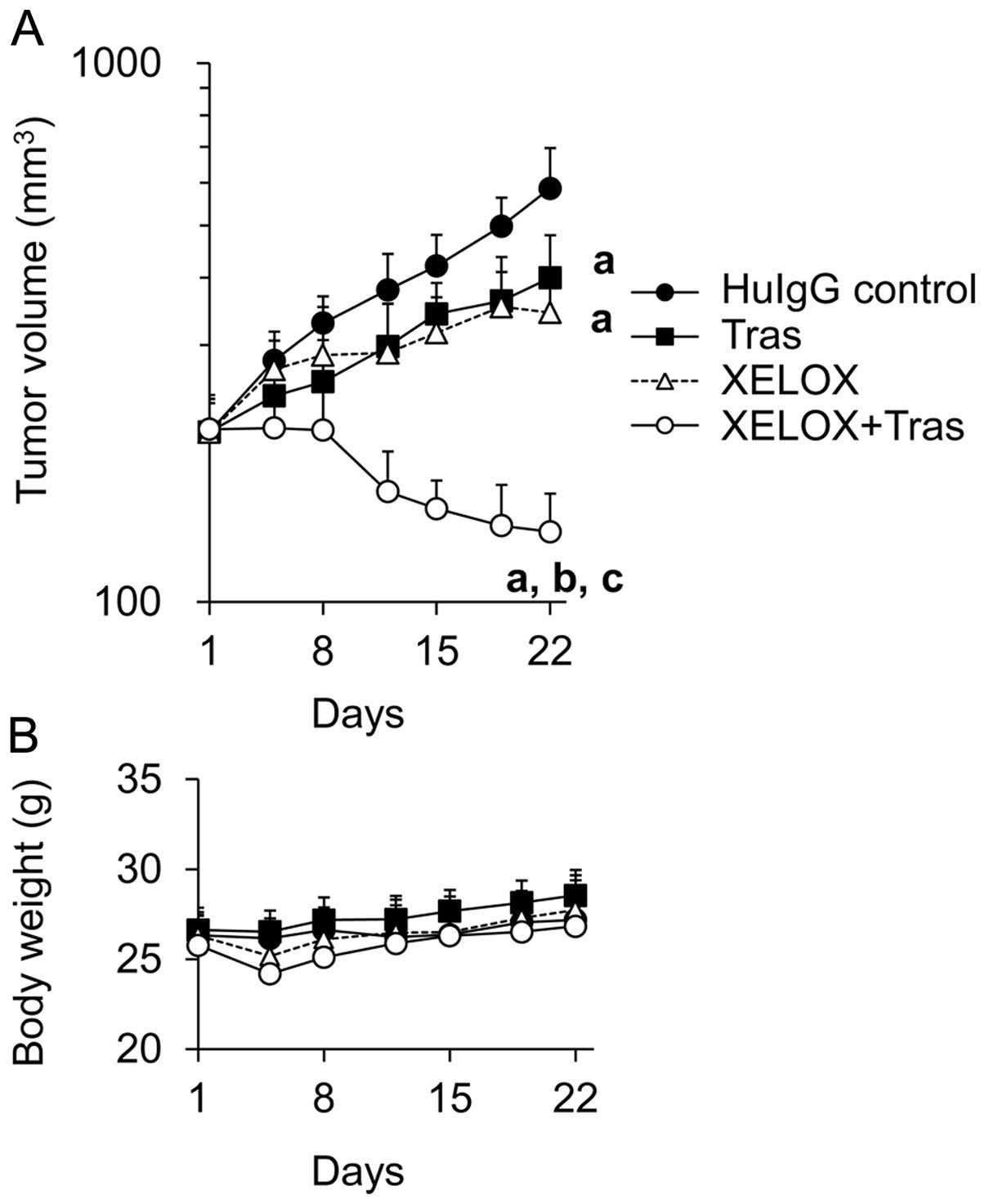
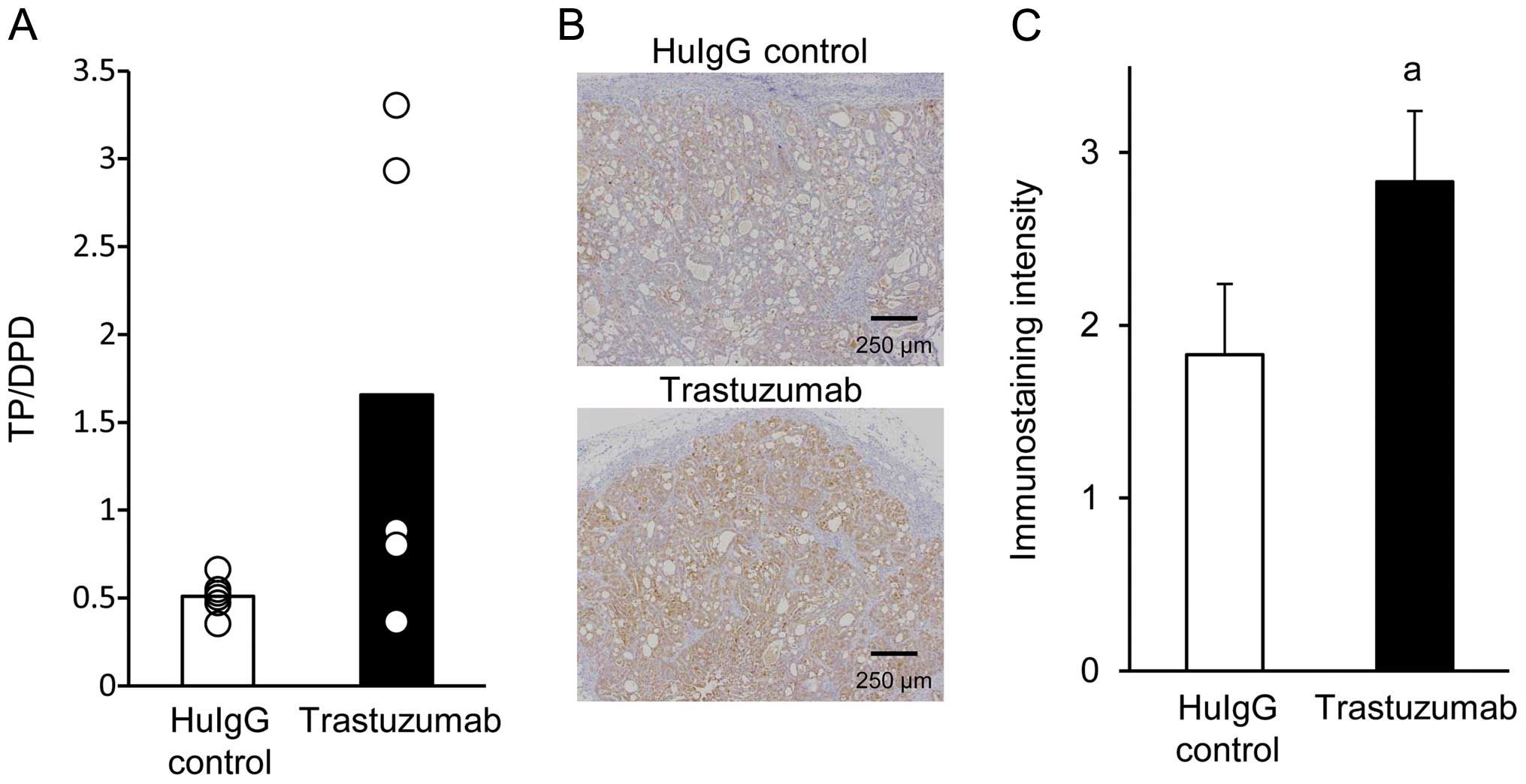
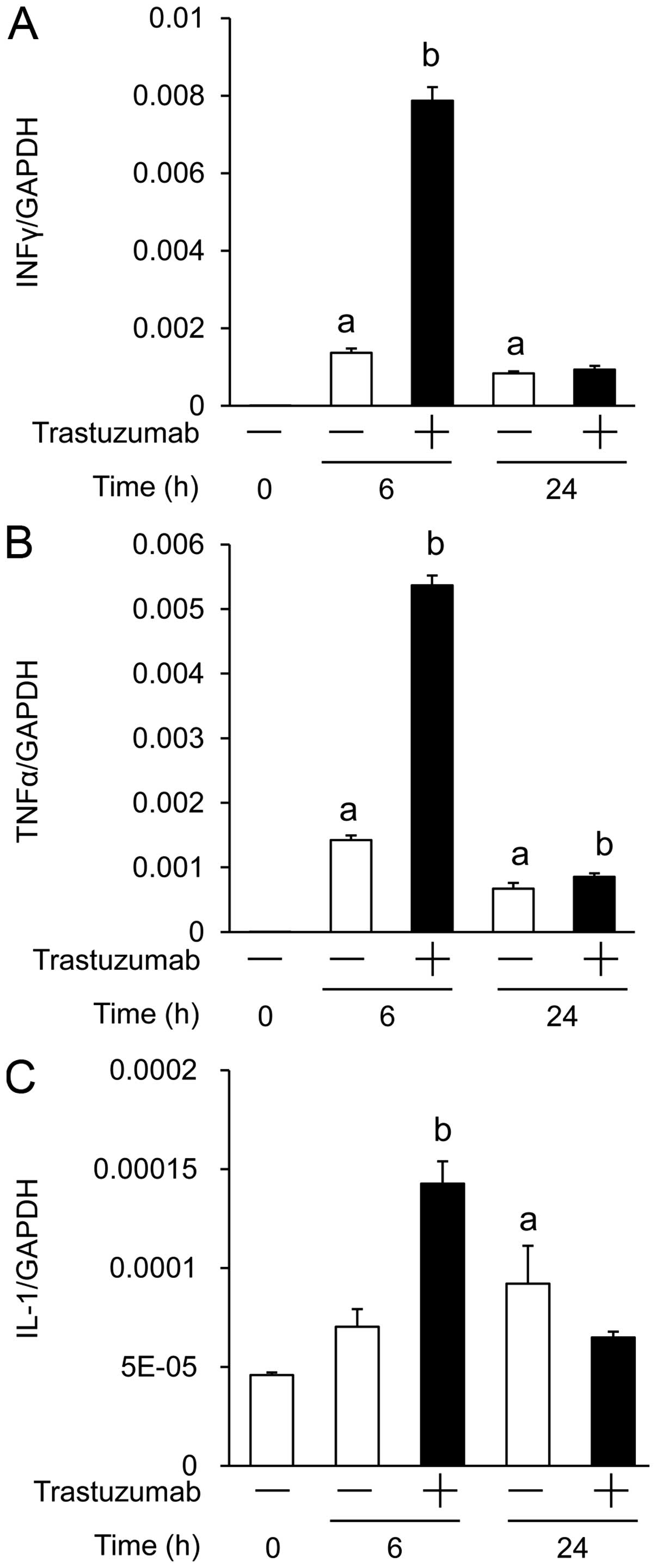
|
1
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sasako M, Saka M, Fukagawa T, Katai H and
Sano T: Surgical treatment of advanced gastric cancer: Japanese
perspective. Dig Surg. 24:101–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunningham D, Allum WH, Stenning SP, et
al: MAGIC Trial Participants: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Starling N, Rao S, et al:
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom: Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
García I, Vizoso F, Martín A, et al:
Clinical significance of the epidermal growth factor receptor and
HER2 receptor in resectable gastric cancer. Ann Surg Oncol.
10:234–241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanner M, Hollmen M, Junttila TT, et al:
Amplification of HER 2 in gastric carcinoma: association with
topoisomerase IIalpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Molina MA, Codony-Servat J, Albanell J, et
al: Trastuzumab (herceptin), a humanized anti-Her2 receptor
monoclonal antibody, inhibits basal and activated Her2 ectodomain
cleavage in breast cancer cells. Cancer Res. 61:4744–4749.
2001.PubMed/NCBI
|
|
10
|
Izumi Y, Xu L, di Tomaso E, Fukumura D and
Jain RK: Tumour biology: Herceptin acts as an anti-angiogenic
cocktail. Nature. 416:279–280. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barok M, Isola J, Pályi-Krekk Z, et al:
Trastuzumab causes antibody-dependent cellular
cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1
breast cancer xenografts despite intrinsic drug resistance. Mol
Cancer Ther. 6:2065–2072. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valabrega G, Montemurro F and Aglietta M:
Trastuzumab: mechanism of action, resistance and future
perspectives in HER2-overexpressing breast cancer. Ann Oncol.
18:977–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Musolino A, Naldi N, Bortesi B, et al:
Immunoglobulin G fragment C receptor polymorphisms and clinical
efficacy of trastuzumab-based therapy in patients with
HER-2/neu-positive metastatic breast cancer. J Clin Oncol.
26:1789–1796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buzdar AU, Valero V, Ibrahim NK, et al:
Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil,
epirubicin and cyclophosphamide chemotherapy and concurrent
trastuzumab in human epidermal growth factor receptor 2-positive
operable breast cancer: An update of the initial randomized study
population and data of additional patients treated with the same
regimen. Clin Cancer Res. 13:228–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Romond EH, Perez EA, Bryant J, et al:
Trastuzumab plus adjuvant chemotherapy for operable HER2-positive
breast cancer. N Engl J Med. 353:1673–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith I, Procter M, Gelber RD, et al: HERA
study team: Two year follow-up of trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer: A randomised
controlled trial. Lancet. 369:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujimoto-Ouchi K, Sekiguchi F, Yasuno H,
Moriya Y, Mori K and Tanaka Y: Antitumor activity of trastuzumab in
combination with chemotherapy in human gastric cancer xenograft
models. Cancer Chemother Pharmacol. 59:795–805. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishikawa T, Utoh M, Sawada N, et al: Tumor
selective delivery of 5-fluorouracil by capecitabine, a new oral
fluoropyrimidine carbamate, in human cancer xenografts. Biochem
Pharmacol. 55:1091–1097. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pentheroudakis G and Twelves C: The
rational development of capecitabine from the laboratory to the
clinic. Anticancer Res. 22:3589–3596. 2002.PubMed/NCBI
|
|
21
|
Schüller J, Cassidy J, Dumont E, et al:
Preferential activation of capecitabine in tumor following oral
administration to colorectal cancer patients. Cancer Chemother
Pharmaco. 45:291–297. 2000. View Article : Google Scholar
|
|
22
|
Ishikawa T, Sekiguchi F, Fukase Y, Sawada
N and Ishitsuka H: Positive correlation between the efficacy of
capecitabine and doxifluridine and the ratio of thymidine
phosphorylase to dihydropyrimidine dehydrogenase activities in
tumors in human cancer xenografts. Cancer Res. 58:685–690.
1998.PubMed/NCBI
|
|
23
|
Yasuno H, Kurasawa M, Yanagisawa M, Sato
Y, Harada N and Mori K: Predictive markers of capecitabine
sensitivity identified from the expression profile of pyrimidine
nucleoside-metabolizing enzymes. Oncol Rep. 29:451–458.
2013.PubMed/NCBI
|
|
24
|
Ishii R, Takiguchi N, Oda K, Koda K and
Miyazaki M: Thymidine phosphorylase expression is useful in
selecting adjuvant chemotherapy for stage III gastric cancer. Int J
Oncol. 19:717–722. 2001.PubMed/NCBI
|
|
25
|
Nishimura G, Terada I, Kobayashi T, et al:
Thymidine phosphorylase and dihydropyrimidine dehydrogenase levels
in primary colorectal cancer show a relationship to clinical
effects of 5′-deoxy-5-fluorouridine as adjuvant chemotherapy. Oncol
Rep. 9:479–482. 2002.PubMed/NCBI
|
|
26
|
Terashima M, Fujiwara H, Takagane A, et
al: Role of thymidine phosphorylase and dihydropyrimidine
dehydrogenase in tumour progression and sensitivity to
doxifluridine in gastric cancer patients. Eur J Cancer.
38:2375–2381. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Endo M, Shinbori N, Fukase Y, et al:
Induction of thymidine phosphorylase expression and enhancement of
efficacy of capecitabine or 5′-deoxy-5-fluorouridine by
cyclophosphamide in mammary tumor models. Int J Cancer. 83:127–134.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sawada N, Ishikawa T, Fukase Y, Nishida M,
Yoshikubo T and Ishitsuka H: Induction of thymidine phosphorylase
activity and enhancement of capecitabine efficacy by taxol/taxotere
in human cancer xenografts. Clin Cancer Res. 4:1013–1019.
1998.PubMed/NCBI
|
|
29
|
Sawada N, Ishikawa T, Sekiguchi F, Tanaka
Y and Ishitsuka H: X-ray irradiation induces thymidine
phosphorylase and enhances the efficacy of capecitabine (Xeloda) in
human cancer xenografts. Clin Cancer Res. 5:2948–2953.
1999.PubMed/NCBI
|
|
30
|
Sawada N, Kondoh K and Mori K: Enhancement
of capecitabine efficacy by oxaliplatin in human colorectal and
gastric cancer xenografts. Oncol Rep. 18:775–778. 2007.PubMed/NCBI
|
|
31
|
Ouchi KF, Yanagisawa M, Sekiguchi F and
Tanaka Y: Antitumor activity of erlotinib in combination with
capecitabine in human tumor xenograft models. Cancer Chemother
Pharmacol. 57:693–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Woynarowski JM, Faivre S, Herzig MC, et
al: Oxaliplatin-induced damage of cellular DNA. Mol Pharmacol.
58:920–927. 2000.PubMed/NCBI
|
|
33
|
Dong N, Jiang W, Li H, Liu Z, Xu X and
Wang M: Triweekly oxaliplatin plus oral capecitabine as first-line
chemotherapy in elderly patients with advanced gastric cancer. Am J
Clin Oncol. 32:559–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu C, Sun Q, Hang X, Zhong B and Wang D:
Multicenter phase II study of capecitabine plus oxaliplatin as a
first-line therapy in Chinese patients with advanced gastric
cancer. Anticancer Drugs. 19:825–831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park YH, Lee JL, Ryoo BY, et al:
Capecitabine in combination with oxaliplatin (XELOX) as a
first-line therapy for advanced gastric cancer. Cancer Chemother
Pharmacol. 61:623–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bang YJ, Kim YW, Yang HK, et al: CLASSIC
trial investigators: Adjuvant capecitabine and oxaliplatin for
gastric cancer after D2 gastrectomy (CLASSIC): A phase 3
open-label, randomised controlled trial. Lancet. 379:315–321. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamashita Kashima Y, Iijima S, Yorozu K,
et al: Pertuzumab in combination with trastuzumab shows
significantly enhanced antitumor activity in HER2-positive human
gastric cancer xenograft models. Clin Cancer Res. 17:5060–5070.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mori K, Hasegawa M, Nishida M, et al:
Expression levels of thymidine phosphorylase and dihydropyrimidine
dehydrogenase in various human tumor tissues. Int J Oncol.
17:33–38. 2000.PubMed/NCBI
|
|
39
|
Nishida M, Hino A, Mori K, Matsumoto T,
Yoshikubo T and Ishitsuka H: Preparation of anti-human thymidine
phosphorylase monoclonal antibodies useful for detecting the enzyme
levels in tumor tissues. Biol Pharm Bull. 19:1407–1411. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Spector NL and Blackwell KL: Understanding
the mechanisms behind trastuzumab therapy for human epidermal
growth factor receptor 2-positive breast cancer. J Clin Oncol.
27:5838–5847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kute TE, Savage L, Stehle JR Jr, et al:
Breast tumor cells isolated from in vitro resistance to trastuzumab
remain sensitive to trastuzumab anti-tumor effects in vivo and to
ADCC killing. Cancer Immunol Immunother. 58:1887–1896. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arnould L, Gelly M, Penault-Llorca F, et
al: Trastuzumab-based treatment of HER2-positive breast cancer: An
antibody-dependent cellular cytotoxicity mechanism? Br J Cancer.
94:259–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Varchetta S, Gibelli N, Oliviero B, et al:
Elements related to heterogeneity of antibody-dependent cell
cytotoxicity in patients under trastuzumab therapy for primary
operable breast cancer overexpressing Her2. Cancer Res.
67:11991–11999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Akiyama S, Furukawa T, Sumizawa T, et al:
The role of thymidine phosphorylase, an angiogenic enzyme, in tumor
progression. Cancer Sci. 95:851–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Albanell J, Codony J, Rovira A, Mellado B
and Gascón P: Mechanism of action of anti-HER2 monoclonal
antibodies: Scientific update on trastuzumab and 2C4. Adv Exp Med
Biol. 532:253–268. 2003. View Article : Google Scholar : PubMed/NCBI
|