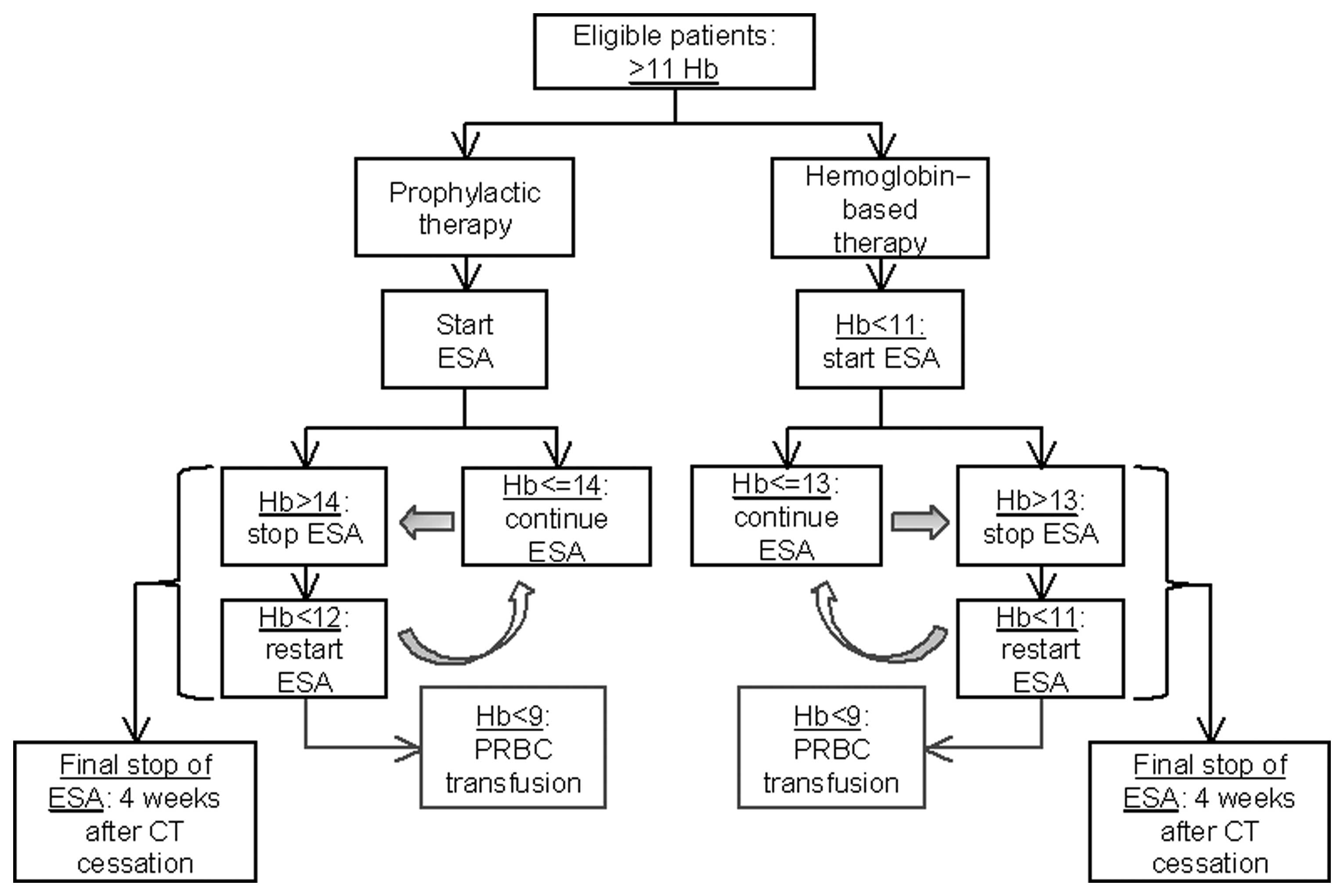
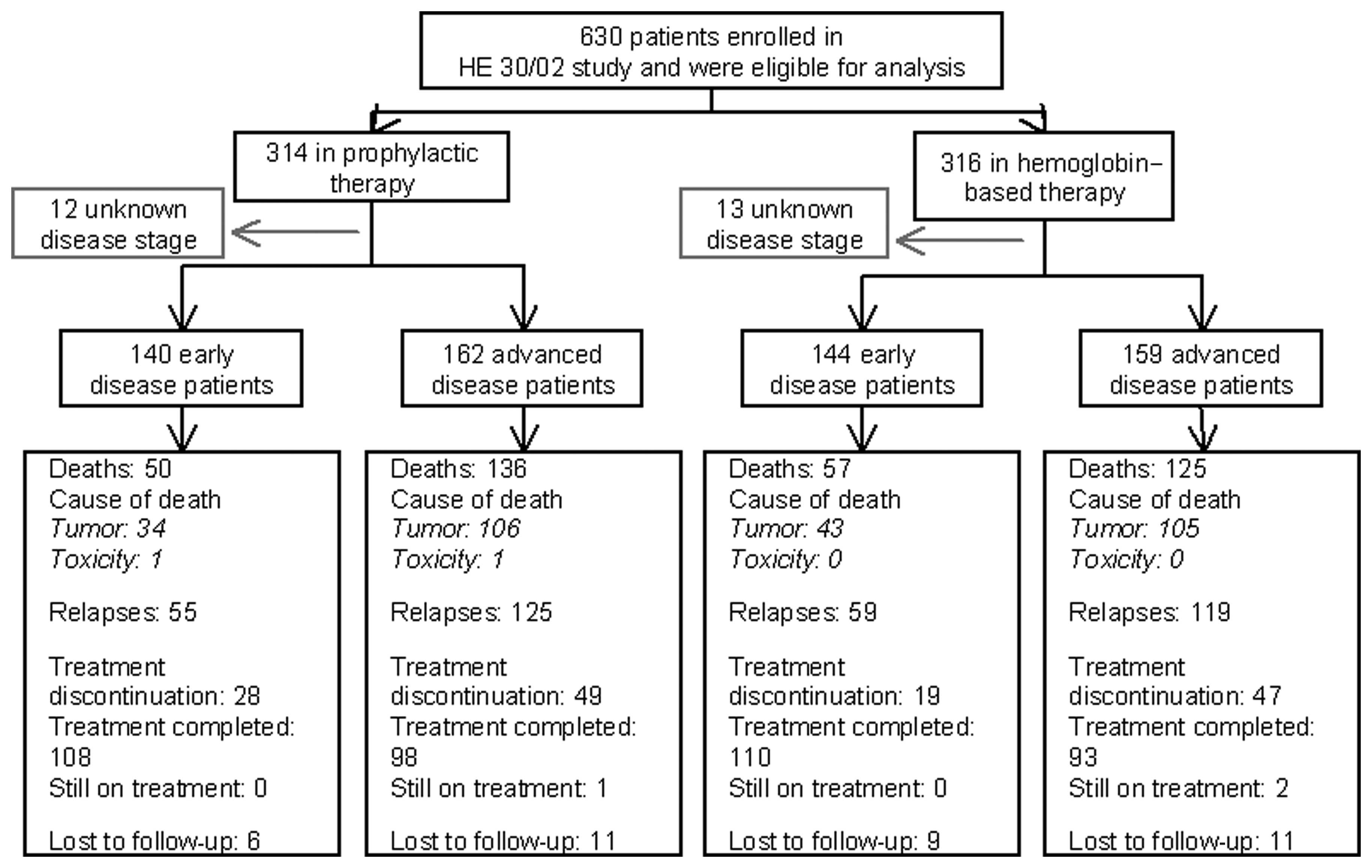
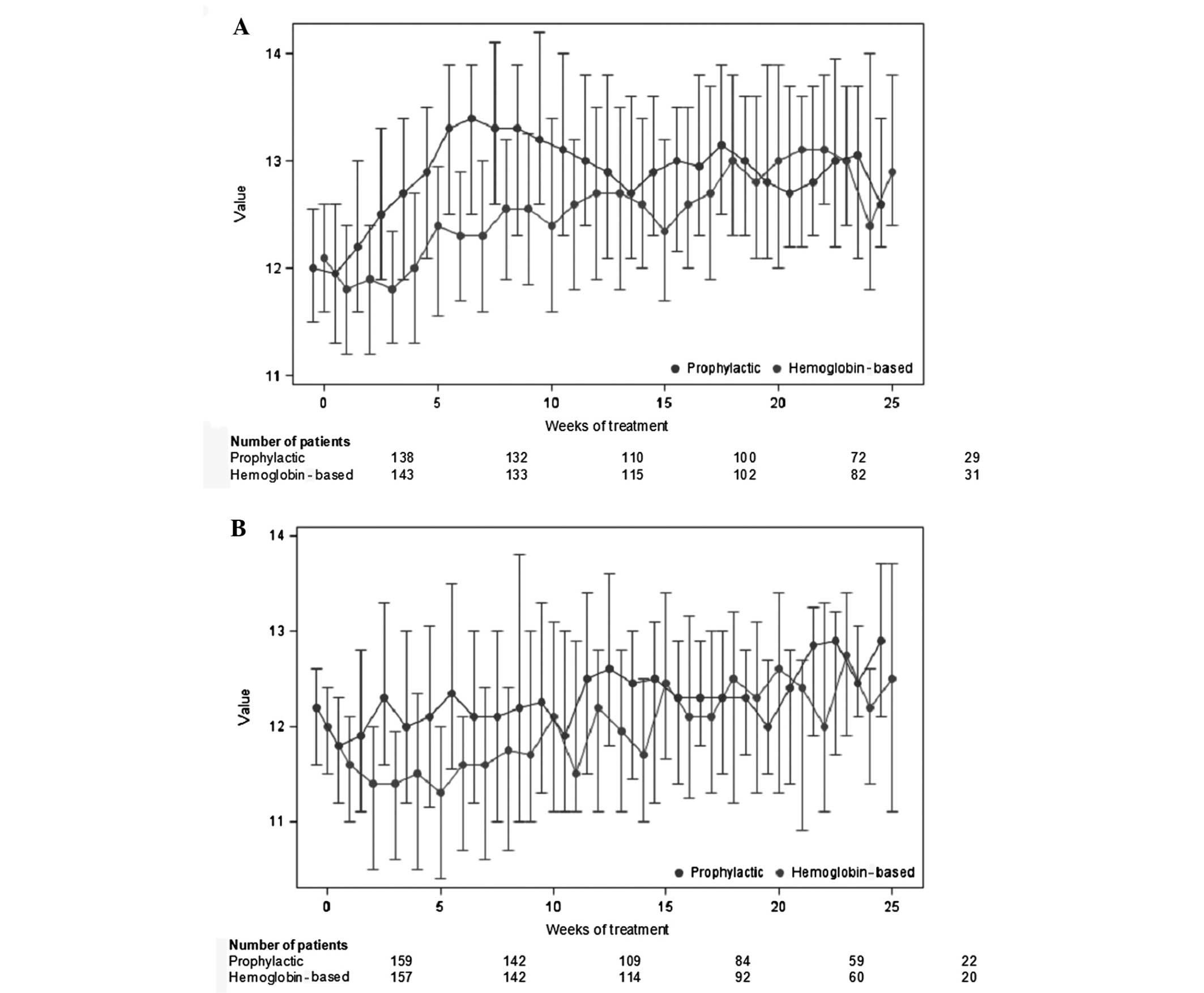
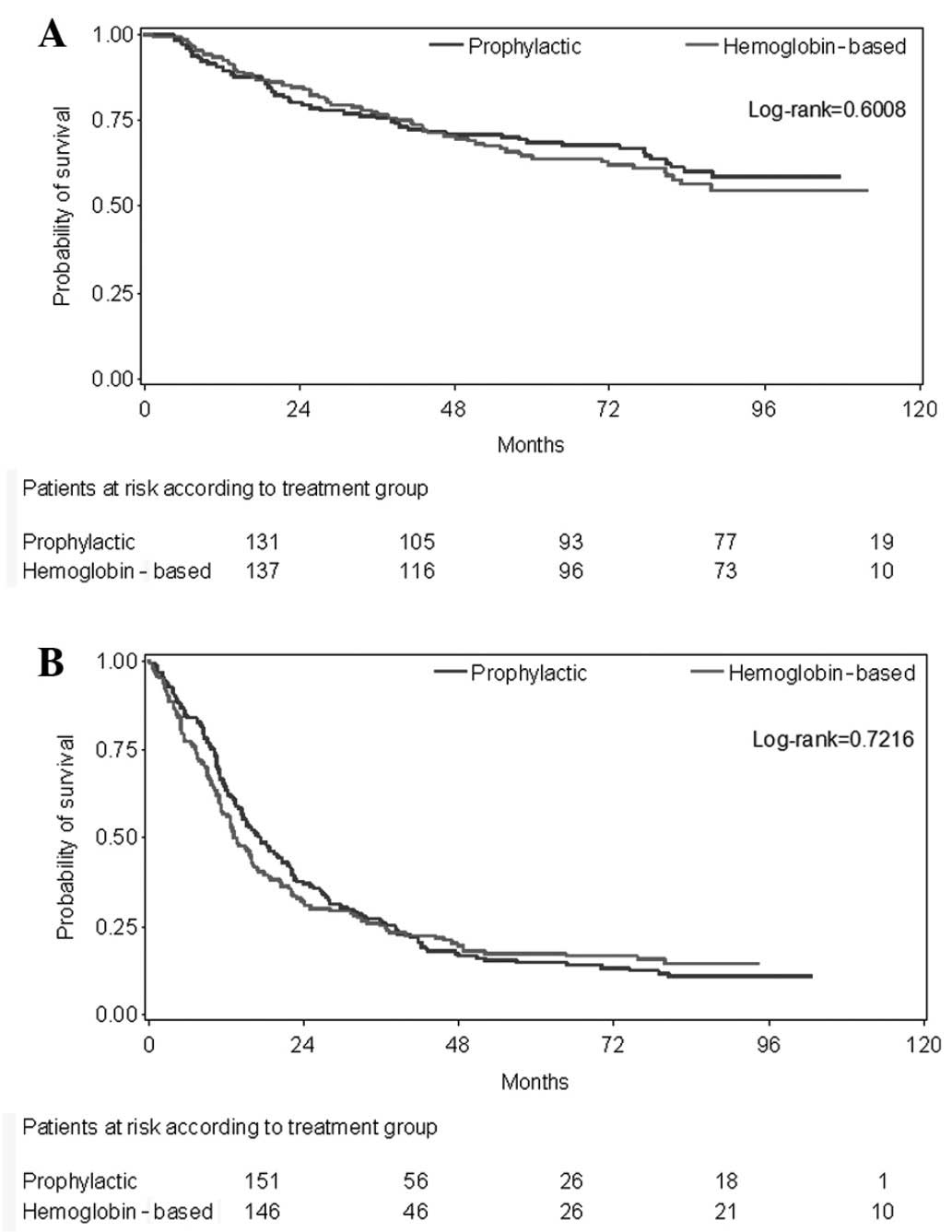
Spandidos Publications style
Mountzios G, Aravantinos G, Alexopoulou Z, Timotheadou E, Matsiakou F, Christodoulou C, Laschos K, Galani E, Koutras A, Bafaloukos D, Bafaloukos D, et al: Lessons from the past: Long-term safety and survival outcomes of a prematurely terminated randomized controlled trial on prophylactic vs. hemoglobin-based administration of erythropoiesis-stimulating agents in patients with chemotherapy-induced anemia. Mol Clin Oncol 4: 211-220, 2016.
APA
Mountzios, G., Aravantinos, G., Alexopoulou, Z., Timotheadou, E., Matsiakou, F., Christodoulou, C. ... Fountzilas, G. (2016). Lessons from the past: Long-term safety and survival outcomes of a prematurely terminated randomized controlled trial on prophylactic vs. hemoglobin-based administration of erythropoiesis-stimulating agents in patients with chemotherapy-induced anemia. Molecular and Clinical Oncology, 4, 211-220. https://doi.org/10.3892/mco.2015.693
MLA
Mountzios, G., Aravantinos, G., Alexopoulou, Z., Timotheadou, E., Matsiakou, F., Christodoulou, C., Laschos, K., Galani, E., Koutras, A., Bafaloukos, D., Linardou, H., Pectasides, D., Varthalitis, I., Papakostas, P., Kalofonos, H. P., Fountzilas, G."Lessons from the past: Long-term safety and survival outcomes of a prematurely terminated randomized controlled trial on prophylactic vs. hemoglobin-based administration of erythropoiesis-stimulating agents in patients with chemotherapy-induced anemia". Molecular and Clinical Oncology 4.2 (2016): 211-220.
Chicago
Mountzios, G., Aravantinos, G., Alexopoulou, Z., Timotheadou, E., Matsiakou, F., Christodoulou, C., Laschos, K., Galani, E., Koutras, A., Bafaloukos, D., Linardou, H., Pectasides, D., Varthalitis, I., Papakostas, P., Kalofonos, H. P., Fountzilas, G."Lessons from the past: Long-term safety and survival outcomes of a prematurely terminated randomized controlled trial on prophylactic vs. hemoglobin-based administration of erythropoiesis-stimulating agents in patients with chemotherapy-induced anemia". Molecular and Clinical Oncology 4, no. 2 (2016): 211-220. https://doi.org/10.3892/mco.2015.693