Introduction
Desmoplastic small round cell tumor (DSRCT) is an
uncommon malignant mesenchymal tumor demonstrating a complex
pattern of simultaneous polyphenotypic differentiation, expressing
proteins associated with epithelial, muscular and neural
differentiation (1). Its various
appellations include intra-abdominal desmoplastic small round cell
tumor, intra-abdominal desmoplastic small cell tumor with divergent
differentiation, polyphenotypic small round cell tumor and
mesothelioblastoma (1). DSRCT was
first described by Gerald and Rosai in 1989 (2). DSRCT has a highly aggressive clinical
course with high risk of local recurrence and distant metastases,
and is automatically assigned as high-grade sarcoma. The patterns
of metastasis of DSRCT are similar to those of the other abdominal
malignancies such as gastrointestinal carcinoma, with both
intraperitoneal and retroperitoneal lymphatic routes being
frequently encountered (1). The
overall prognosis is poor due to the aggressive nature of the
disease. Despite aggressive treatment, the 5-year survival rate is
<15% (3).
Sister Mary Joseph nodule is a rare and peculiar
physical sign that is encountered in 1–3% of patients with
intra-abdominal malignancy (4). It
is an umbilical intraperitoneal metastasis from an underlying
extensive intra-abdominal malignancy. Commonly encountered primary
tumors associated with umbilical metastasis include stomach, ovary,
colorectum and pancreas. Umbilical metastasis presenting as Sister
Mary Joseph nodule from DSRCT is extremely rare (5–7). The
present study reports a rare case of Sister Mary Joseph nodule
caused by metastatic DSRCT.
Case report
Clinical summary
An 18-year-old Thai man presented with an umbilical
nodule and a gradual enlargement of his abdominal mass for 4
months. The patient had no history of significant illness in the
past. The patient did not drink alcohol or smoke, and had no
history of tuberculosis and cancer among his family members.
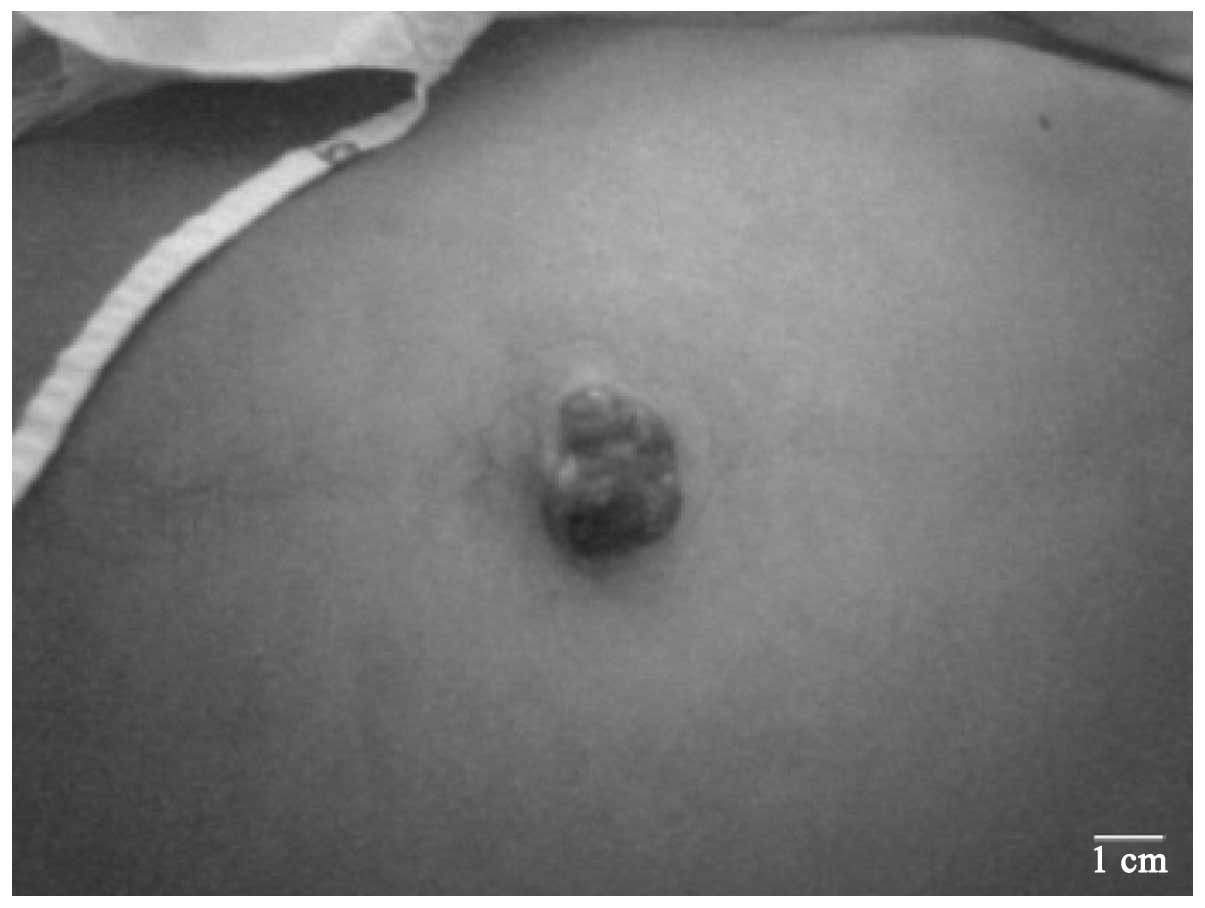
Physical examination revealed a well-defined, firm violaceous mass,
measuring 2.3 cm in its greatest dimension, located at the
umbilicus (Fig. 1). There was
neither superficial vein dilatation nor evidence of inflammation.
The abdomen exhibited an ill-defined firm mass measuring 15×13×10
cm. The cervical and inguinal lymph nodes could not be palpated. An
evaluation of the right lung revealed decreased breath sound.
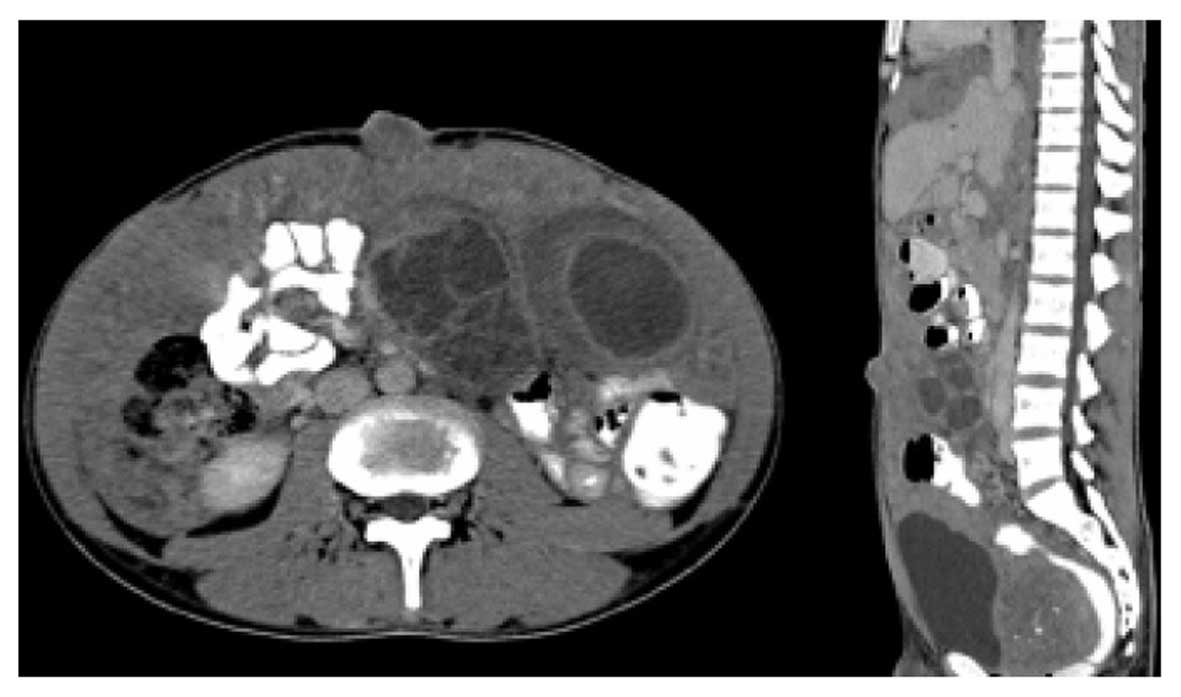
Computed tomography (CT, Multidetector CT: Aquilion CX, Toshiba
Medical Systems Corporation, Tokyo, Japan) of the abdomen revealed
a diffuse peritoneal mass (Fig. 2)
and extensive intraperitoneal seeding, and multiple
intra-abdominal, pelvic and bilateral inguinal lymphadenopathies,
with multiple bilobar hepatic metastases. Left ureteric obstruction
with hydronephrosis and partial colonic obstruction were detected.
The patient underwent a fine needle aspiration (FNA) of the ascitic
fluid and right pleural effusion, and a minilaparotomy with
incisional biopsy of the intra-abdominal mass and Sister Mary
Joseph nodule. The pathological diagnosis was DSRCT, histological
grade 3, high grade, Gilly classification 4, classified as stage
IV. The patient achieved a stable disease following four courses of
VDC/IE regimen of chemotherapy, which consisted of vincristine (1.4
mg/m2), doxorubicin (75 mg/m2) and
cyclophosphamide (1,200 mg/m2) alternating with
ifosfamide (9 gm/m2) and etoposide (500
mg/m2). The patient desired no further treatment.
Finally, the patient succumbed to the disease 6 months following
the diagnosis of DSRCT with systemic metastasis. No autopsy was
performed.
The present study was approved by the Committee on
Human Rights Related to Researches involving Human Subjects
(Faculty of Medicine, Ramathibodi Hospital, Mahidol University;
ID05-51-32).
Pathological findings
FNA of the ascitic fluid and right pleural effusion
revealed cohesive groups of tumor cells, revealing the presence of
small-sized cells with round, slightly pleomorphic nuclei with
inconspicuous nucleoli, and a moderate amount of cytoplasm without
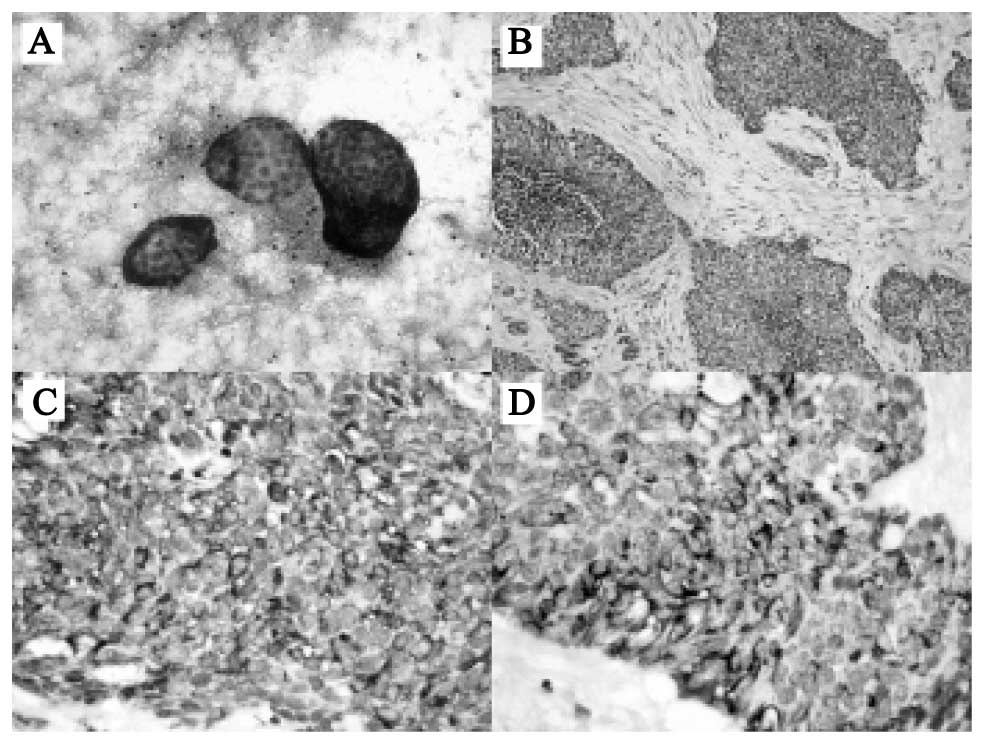
definitive differentiation. The histopathology of the
intra-abdominal mass and Sister Mary Joseph nodule revealed
malignant small, round blue cells embedded in a dense desmoplastic
stroma (Fig. 3A and B). The cells
exhibited vesicular nuclei, with scant cytoplasm and numerous
mitoses. Neither a glandular structure nor rosette formation was
detected. The intervening stroma was negligible in several areas,
or abundant and densely collagenous in others. No vascular
proliferation was identified. There were numerous tumor emboli in
the lymphatic channels. The immunocytohistochemical stains for
cytokeratin (AE1/AE3) (clone AE1/3; 1/100 dilution), epithelial
membrane antigen (EMA) (clone E29; 1/100 dilution), desmin (clone
D33; 1/200 dilution) (all from Dako, Carpinteria, CA, USA),
neuron-specific enolase (NSE) (clone 5E2; 1/100), Wilms' tumor 1
(WT1) (clone WT49; 1/40) (both from Leica, Mannheim, Germany),
vimentin (clone Vim3B4; 1/200 dilution; Dako), CD56 (clone 1B6;
1/200; Leica), CD99 (clone 12E7; 1/75; Dako) and SWI/SNF-related
matrix-associated actin-dependent regulator of chromatin subfamily
B member 1 (SMARCB1/INI1) (clone MRQ-27, optimally diluted; Ventana
Medical Systems, Inc., Tucson, AZ, USA) were positive in the
malignant small round cells (Fig. 3C and
D). The MIB-1 (polyclonal; 1/100; Dako) index was 80%. The
tumor cells were immunonegative for chromogranin A (clone DAK-A3;
1/100), synaptophysin (polyclonal; 1/100), leukocyte common antigen
(LCA) (clone 2B11; 1/100), CD3 (clone F7.2.38; 1/50) and CD20
(clone L26; 1/200) (all from Dako).
Discussion
DSRCT is an uncommon malignant mesenchymal neoplasm
composed of small round tumor cells associated with prominent
stromal desmoplasia and polyphenotypic differentiation. The average
age at presentation occurs principally during the second to third
decade, with a range of 6 to 54 years (1,8). This
tumor shows a male predilection of ~10:1 (8). DSRCT is frequently found in the
abdominal cavity, although cases with involvement of the thoracic
cavity, paratesticular area, kidney, head and neck region have been
reported (1,8–10). The
most frequently presenting symptoms of abdominal DSRCT are vague
abdominal pain, gradual enlargement of the abdominal mass,
abdominal distension, abdominal pain, weight loss and other
symptoms associated with obstruction of the intestinal or urinary
tract (5–10). The most common site of metastasis is
the regional abdominal lymph node. Distant metastases usually
involve the lung, liver and bone (1,8). The
routine initial laboratory investigations and serum tumor markers
are non-contributory. The most useful diagnostic tool is the CT
scan, which reveals a characteristic pattern of multiple
intra-abdominal masses without any apparent association with the
primary organ. FNA of the affected organs may allow early
recognition of malignant soft tissue tumors. Core needle or opened
biopsy is required for an accurate immunohistopathological
diagnosis. Identification and confirmation of the primary tumor is
important in order to facilitate treatment.
The macroscopic findings of DSRCT are solid, firm
and multilobulated masses, with a gray-white cut surface. The tumor
size ranges vary from 1 to 40 cm, with an average size of 10 cm
(1,8,10). The
microscopic findings demonstrate solid sheets, large nests, small
clumps, or cords of cohesive, small, round, ovoid or spindled cells
lying in a hypocellular, desmoplastic, collagenous stroma. The
tumor cells are characterized by small, round, oval or elongated
hyperchromatic nuclei, clumped chromatin, inconspicuous nucleoli
and ill-defined, lightly eosinophilic cytoplasm with an indistinct
cytoplasmic border.
The differential diagnoses of primary malignant
small round cell tumor of the abdomen include lymphoma, primitive
neuroectodermal tumor (PNET)/Ewing sarcoma, small cell carcinoma,
rhabdomyosarcoma, neuroblastoma, Wilms' tumor and extrarenal
rhabdoid tumor (8–13). Lymphoma histologically reveals
atypical lymphocytes infiltrating and replacing normal structure.
Negative results of immunohistochemical stains for LCA, CD3 and
CD20 may be helpful in excluding lymphoma. The PNET/Ewing sarcoma
may be histologically indistinguishable from DSRCT. In the present
case study, the immunohistochemical stains were positive for
desmin, vimentin, keratin and WT1, which are typically negative for
PNET/Ewing sarcoma (10–13). Small cell carcinoma demonstrates many
cytological and histological similarities to DSRCT. Clinically,
small cell carcinoma is associated with a much older patient
population, and usually originates in the lung. On histological
examination, a desmoplastic stroma is not identified to be a
feature of small cell carcinoma. However, small cell carcinoma
demonstrates immunoreactivity with epithelial markers, including
cytokeratin and EMA, but is negative for myogenic markers, such as
desmin. Rhabdomyosarcoma may reveal small blue cells arranged in
nests or sheets. Immunohistochemically, rhabdomyosarcoma is
positive for muscle markers, but usually negative for cytokeratin
and neural markers, including NSE (6–9).
Neuroblastoma and Wilms' tumor also share several histological
features with DSRCT, although they occur predominantly in young
children and are typically associated with adrenal and renal
masses, respectively. Extrarenal rhabdoid tumor is characterized by
loss of SMARCB1/INI1, as revealed by immunohistochemistry.
Sister Mary Joseph nodules are rare physical signs
that are encountered in 1–3% of patients with intra-abdominal
and/or pelvic malignancy (4).
Commonly encountered primary tumors associated with umbilical
metastasis include stomach, ovary, endometrium, large intestine and
pancreas. The occurrence of a Sister Mary Joseph nodule
metastasizing from the DSRCT is very rare. Table I compares the present rare case of
DSRCT with three reported cases of Sister Mary Joseph nodule caused
by metastatic DSRCT that have been previously described in the
literature (5–7). The presence of Sister Mary Joseph
nodule often means a poor prognosis, with a median survival time of
6 months for metastatic DSRCT. Metastases to the umbilicus occur
predominantly through the lymphatic and venous channels, although
contiguous extension from the peritoneal surface and embryonic
remnant has been reported (4). In
the present case study, the presence of tumor nests on the
peritoneal surface and in numerous lymphatic channels indicated
that more than one mechanism could have been involved.
 | Table I.Summary of four cases of Sister Mary
Joseph nodule caused by metastatic desmoplastic small round cell
tumor. |
Table I.
Summary of four cases of Sister Mary
Joseph nodule caused by metastatic desmoplastic small round cell
tumor.
| Authors | Gender | Age (years) | Symptoms | Duration | Tumor size | Radiotherapy | Chemotherapy | Systemic
metastasis | Survival | Refs. |
|---|
| Albano et
al | F | 12 | Decreased appetite,
vomiting, weight loss, Sister Mary Joseph nodule | 1 month | Multiple peritoneal
masses | NP | VDC/IE | Ovary, liver | Eight months
following diagnosis, the patient is clinically well with stable
disease | (5) |
| Abdulqawi et
al | M | 28 | Colicky central
abdominal pain, Sister Mary Joseph nodule | 2 weeks | 5.5 cm, with several
peritoneal deposits | NP | Systemic
chemotherapy | NA | NA | (6) |
| Doros et
al | M | 14 | Sister Mary Joseph
nodule | 2–3 weeks | Multiple peritoneal
masses | 30 Gy whole abdomen
radiation with boosts for a total of 45 Gy to right flank and
umbilicus and 36 Gy to the inguinal region | VDC/IE | NA | Succumbed to
mortality during the course of treatment | (7) |
| Larbcharoensub et
al | M | 18 | Sister Mary Joseph
nodule, abdominal mass | 4 months | 15 cm, with multiple
peritoneal seeding | NP | VDC/IE | Liver | Succumbed to
mortality after 6 months following diagnosis during the course of
treatment | The present
study |
Reports of DSRCT have identified reciprocal
translocation (11;22)(p13;q12), resulting in a fusion gene between
exon 7 of the Ewing sarcoma RNA-binding protein 1 (EWSR1)
gene on chromosome 22 and exon 8 of the WT1 suppressor gene
on chromosome 11 (1). The resultant
chimeric protein is considered to be a transcriptional activator
that fails to suppress tumor cell growth. The EWSR1-WT1
chimeric transcript also induces expression of endogenous
platelet-derived growth factor-A (PDGFA) (14,15).
PDGFA is a potent fibroblastic growth factor that could contribute
to one of the most distinctive histological features of DSRCT: The
dense fibrous or desmoplastic stroma, consisting of collagen fibers
and an important component of elongated mesenchymal cells with
features of fibroblasts or myofibroblasts (14). In addition, studies of the EWSR1-WT1
aberrant transcription factor have revealed deregulation of several
target genes, including interleukin 2 receptor β (IL-2Rβ),
BAI1-associated protein 3 (BAIAP3), myelodysplasia/myeloid
leukemia factor 1 (MLF1), T-cell acute lymphoblastic
leukemia-associated antigen 1 (TALLA-1) and leucine-rich
repeat containing 15 (LRRC15) (15).
The pathogenesis of DSRCT has yet to be fully
elucidated. The possibility that DSRCT is of mesothelial origin has
been suggested due to the diffuse peritoneal pattern of spread, the
presence of epithelial differentiation in the tumor cell, and the
fact that fetal mesothelium co-expresses keratin and desmin
(16). The small cell mesothelioma
also frequently expresses NSE. It has been suggested that DSRCT may
be blastomas arising from the lateral mesoderm or intraembryonic
coelom (17). Moreover, the serosal
lining of body cavities of splanchnic lateral mesoderm, the most
common site of DSRCT, has high transient fetal expression of the
WT1 gene (15). However,
EWSR1-WT1 is typically expressed in tissues derived from the
intermediate mesoderm (1). Further
molecular study in DSRCT patients is warranted, and has important
implications for the study of the pathogenesis of disease.
Radical surgery and adjuvant or neoadjuvant
chemotherapy remain the cornerstone of the treatment of DSRCT.
However, a complete resection is rarely possible, since DSRCT tends
to be large, multifocal and invasive. Several chemotherapy
regimens, including alkylating agents and an aggressive
chemotherapy regimen followed by myeloablative chemotherapy and
autologous stem cell rescue, have been tried, although the result
failed to demonstrate a clear benefit for autologous stem cell
transplant in improving the clinical outcome (18). Current treatment protocols include
multiagent chemotherapy and adjuvant surgery and radiotherapy
(19). The emphasis is on achieving
a complete and durable response. DSRCT has a highly aggressive
clinical course, with a high risk of local recurrence and distant
metastases. The median survival time is ~17–25 months (18). Improved survival rates are associated
with complete resection of the tumor. Poor survival rates, with a
median survival time of 6 months, are associated with Sister Mary
Joseph nodule caused by metastatic DSRCT (5–7). Until
more effective forms of treatment are found, the authors of this
case study recommend multimodality treatment, including
chemotherapy, surgery and radiotherapy.
In conclusion, DSRCT must be considered in the
differential diagnosis of metastatic tumor of the umbilicus forming
Sister Mary Joseph nodule. The application of an
immunocytohistochemical investigation, in conjunction with the
clinical, radiological and histopathological findings, may assist
in making the diagnosis, leading to the appropriate treatment.
References
|
1
|
Antonescu CR and Ladanyi M: Desmoplastic
small round cell tumourWorld Health Organization (WHO)
Classification of Tumours of Soft tissue and Bone. Pathology and
Genetics. Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: 5.
4th. IARC Press; Lyon: pp. pp225–pp227. 2013
|
|
2
|
Gerald WL and Rosai J: Case 2.
Desmoplastic small cell tumor with divergent differentiation.
Pediatr Pathol. 9:177–183. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lal DR, Su WT, Wolden SL, Loh KC, Modak S
and La Quaglia MP: Results of multimodal treatment for desmoplastic
small round cell tumour. J Pediatr Surg. 40:251–255. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galvan VG: Sister Mary Joseph's nodule.
Ann Intern Med. 128:4101998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Albano EA and Kanter J: Images in clinical
medicine. Sister Mary Joseph's nodule. N Engl J Med. 352:19132005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdulqawi R, Ahmad S and Ashawesh K: A
rare cause of Sister Mary Joseph's nodule. Swiss Med Wkly.
137:559–560. 2007.PubMed/NCBI
|
|
7
|
Doros L, Kaste SC and Rodriguez-Galindo C:
Sister Mary Joseph's nodule as presenting sign of a desmoplastic
small round cell tumor. Pediatr Blood Cancer. 50:388–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lae ME, Roche PC, Jin L, Lloyd RV and
Nascimento AG: Desmoplastic small round cell tumor: A
clinicopathologic, immunohistochemical, and molecular study of 32
tumors. Am J Surg Pathol. 26:823–835. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hassan I, Shyyan R, Donohue JH, Edmonson
JH, Gunderson LL, Moir CR, Arndt CA, Nascimento AG and Que FG:
Intraabdominal desmoplastic small round cell tumors: A diagnostic
and therapeutic challenge. Cancer. 104:1264–1270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang F: Desmoplastic small round cell
tumors: Cytologic, histologic, and immunohistochemical features.
Arch Pathol Lab Med. 130:728–732. 2006.PubMed/NCBI
|
|
11
|
Barnoud R, Sabourin JC, Pasquier D,
Ranchère D, Bailly C, Terrier-Lacombe MJ and Pasquier B:
Immunohistochemical expression of WT1 by desmoplastic small round
cell tumor: A comparative study with other small round cell tumors.
Am J Surg Pathol. 24:830–836. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Devoe K and Weidner N:
Immunohistochemistry of small round-cell tumors. Semin Diagn
Pathol. 17:216–224. 2000.PubMed/NCBI
|
|
13
|
Arnold MA, Schoenfield L, Limketkai BN and
Arnold CA: Diagnostic pitfalls of differentiating desmoplastic
small round cell tumor (DSRCT) from Wilms tumor (WT): Overlapping
morphologic and immunohistochemical features. Am J Surg Pathol.
38:1220–1226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SB, Kolquist KA, Nichols K, Englert C,
Maheswaran S, Ladanyi M, Gerald WL and Haber DA: The EWS-WT1
translocation product induces PDGFA in desmoplastic small
round-cell tumour. Nat Genet. 17:309–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goodman KA, Wolden SL, La Quaglia MP and
Kushner BH: Whole abdominopelvic radiotherapy for desmoplastic
small round-cell tumor. Int J Radiat Oncol Biol Phys. 54:170–176.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hurlimann J: Desmin and neural marker
expression in mesothelial cells and mesotheliomas. Hum Pathol.
25:753–757. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeoh G, Russell P, Wills EJ and Fleming S:
Intra-abdominal desmoplastic small round cell tumor. Pathology.
25:197–202. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Forlenza CJ, Kushner BH, Kernan N, Boulad
F, Magnan H, Wexler L, Wolden SL, LaQuaglia MP and Modak S:
Myeloablative chemotherapy with autologous stem cell transplant for
desmoplastic small round cell tumor. Sarcoma. 2015:2691972015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kallianpur AA, Shukla NK, Deo SV, Yadav P,
Mudaly D, Yadav R and Palaniappan RM: Updates on the multimodality
management of desmoplastic small round cell tumor. J Surg Oncol.
105:617–621. 2012. View Article : Google Scholar : PubMed/NCBI
|