Introduction
Hepatocellular adenoma (HA) is a benign tumor
usually associated with oral contraceptive intake, glycogen storage
disease (GSD) type I and III, and a history of excess androgen
exposure (1–4). Malignant transformation of HA is
relatively rare and has been reported to be associated with
dysregulation of the β-catenin pathway (2,5,6). Nuclear translocation and accumulation
of β-catenin is induced by a dysregulation, such as a mutation of
exon 3 (7), and may be detected by
immunohistochemistry. The presence of bone marrow metaplasia in HA
is an uncommon histological characteristic, with only 2 cases of HA
with bone marrow metaplasia reported to date (8,9).
We herein report a case with increased levels of
proteins induced by des-gamma-carboxy prothrombin (DCP), resected
hepatocellular carcinoma (HCC) and HA with bone marrow metaplasia
arising in a patient with GSD-I.
Case report
History
A 46-year-old woman was diagnosed with GSD-I during
childhood. From that time onwards, she was followed up in a local
hospital and received treatment with atorvastatin and allopurinol.
At the age of 42 years, the patient consulted a physician at the
Saiseikai Karatsu Hospital (Karatsu, Japan), as she continued to
experience developmental disorders, such as short stature (135 cm)
and low weight (36 kg), but was asymptomatic, apart from abdominal
enlargement. The liver edge was palpable 6–7 cm below the right
costal margin. The patient had no other risk factors for liver
tumors, such as hepatitis B or C viral infection, alcohol use, or
autoimmune disease.
Liver function and tumor markers
Laboratory data at the first consult revealed
elevated values of aspartate aminotransferase (96 IU/l), alanine
aminotransferase (69 IU/l), alkaline phosphatase (700 U/l),
gamma-glutamyl transpeptidase (2610 IU/l) and DCP (421 mAU/ml).
Serum total bilirubin, prothrombin time and α-fetoprotein (AFP)
were all within normal limits. Until surgical treatment was
performed, while AFP remained within normal limits, DCP gradually
increased, as shown in Fig. 1.
Imaging
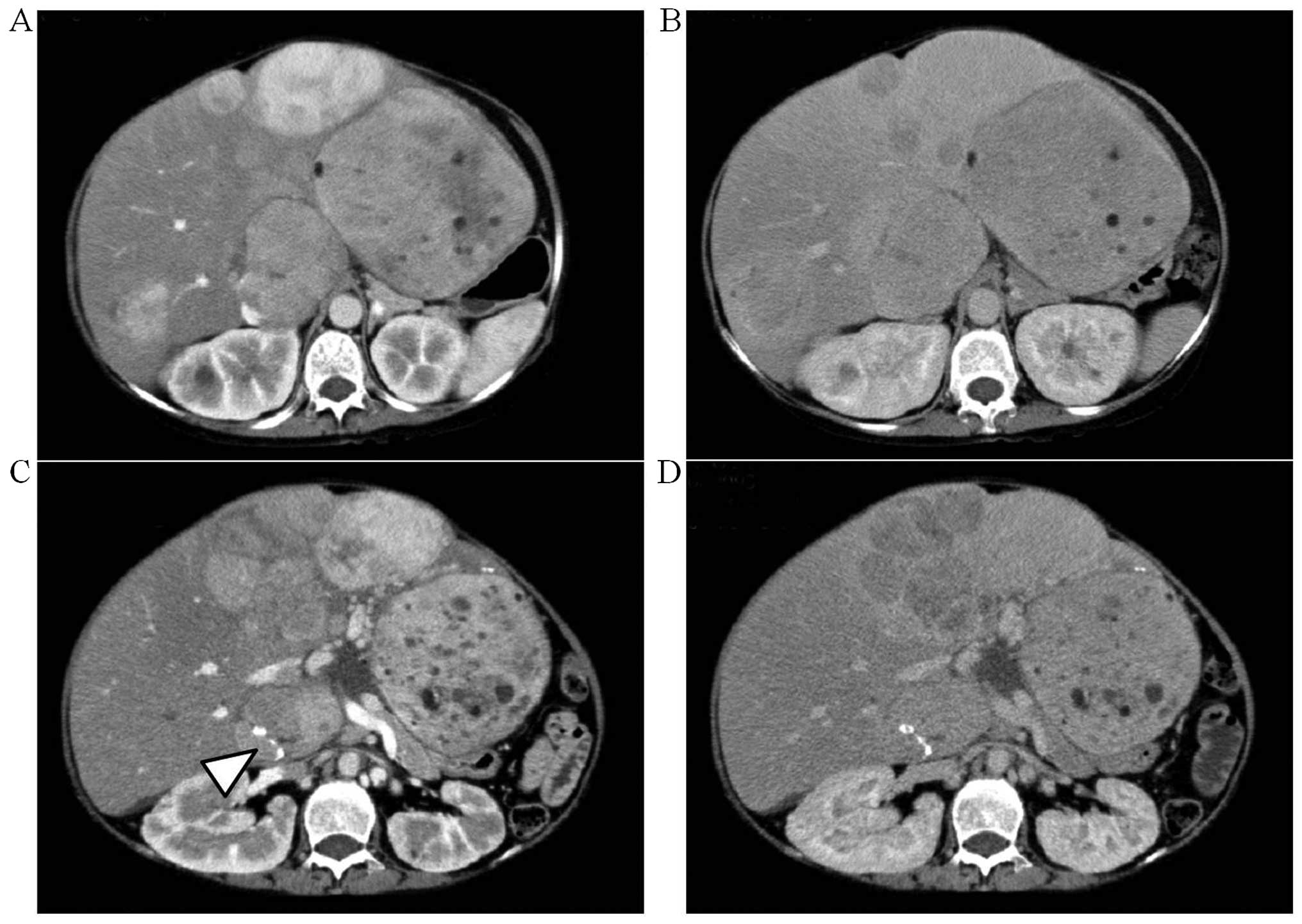
A computed tomography (CT) examination at the first
consult revealed relatively well-circumscribed tumors of various
sizes (Fig. 2A and B) exhibiting
heterogeneous enhancement during the early phase. During the
delayed phase, some of the tumors in the lateral segment or the
right hepatic lobe exhibited isodensity or low density compared
with the surrounding liver parenchyma, whereas the small tumors in
the medial segment exhibited low density. These imaging findings
were compatible with the characteristics of HA. The size and number
of tumors in the medial segment had significantly increased when
compared to the CT from 33 months earlier (Fig. 2C and D). In addition, calcification
was found in the tumor of S1.
Surgical treatment
Due to the gradual increase in tumor size and the
value of DCP (maximum, 10,100 mAU/ml) and irregular tumor
appearance on CT imaging, malignant transformation of HA was
strongly suspected. According to the indication for hepatic
resection, surgery was recommended, but the patient refused this
treatment option for 1 year; when surgery was eventually performed,
she underwent extended left lobectomy. Following hepatic resection,
DCP returned to normal (28 mAU/ml).
Pathological findings
The resected left lobe weighed 2,733 g. The tumors
were divided into four components. In brief, the cut surfaces
revealed a dark green tumor (4.7×3.1 cm) in S1, a dark green tumor
(10.0×8.5 cm) in the lateral segment, a gray colored tumor (6.3×5.0
cm) in the lateral segment, and multiple pale yellow or white
confluent nodules (8.2×7.2 cm) in the medial segment, as shown in
Fig. 3. Smaller pale yellow or white
nodules were also observed. All the tumors were encapsulated by
fibrous bands.
Microscopically, all the tumors, apart from the pale
yellow or white masses, exhibited proliferation of small to
occasionally large hepatocytes, with clear or pale eosinophilic
cytoplasm and mild atypia, surrounded by numerous abnormal arterial
vessels and no portal tract (Fig. 4A and
B). These characteristics indicated HA. It was particularly
worth noting that bone marrow tissue with bone trabeculae and three
series of hematopoietic cells, namely erythroblasts, myeloblasts
and megakaryocytes, was also present in the tumor of S1 (Fig. 4C and D).
The pale yellow or white tumors in the medial
segment exhibited increased cell density and structural atypia.
These tumor cells transformed into atypical cells with relatively
enlarged hyperchromatic nuclei and increased nuclear-cytoplasmic
ratio (Fig. 4E and F). Intrahepatic
metastases were also present in the form of scattered small nodules
consisting of atypical cells. These characteristics indicated
well-to-moderately differentiated HCC, accompanied by HA. In
addition, nuclear accumulation of β-catenin was observed in the
carcinoma cells (Fig. 4G), but not
in the adenoma cells (data not shown) by immunohistochemistry using
anti-β-catenin mouse monoclonal antibody (dilution, 1:200; cat. no.
610154; BD Transduction Laboratories, Lexington, KY, USA). However,
no mutation was detected in exon 3 of β-catenin by direct
sequencing (data not shown).
The non-tumorous liver comprised hepatocytes with
predominantly clear or pale cytoplasm, reflecting the accumulation
of glycogen and glycogenated nuclei separated by portal-portal or
portal-central thin bridging fibrosis (Fig. 4H). This characteristic was compatible
with GSD-I.
Discussion
HA is a relatively rare benign liver tumor that
mainly occurs in women who use oral contraceptives (1,2). GSD-I
is a rare metabolic disorder caused by deficient activity of
glucose-6-phosphatase, an enzyme necessary for gluconeogenesis and
glycogenolysis (10). GSD-I has been
reported to be a cause of HA (3). An
adenoma-carcinoma progression sequence may be expected in HCC,
similar to colon cancer (11).
Bone marrow metaplasia in HA is an unusual
characteristic, with only 2 cases reported to date (8,9).
Romacciato et al reported a case of HA where the bone marrow
contained erythroblasts and myeloblasts, but not megakaryocytes. To
the best of our knowledge, this is the first case report of an HA
with absolute bone marrow metaplasia, producing three series of
hematopoietic cells, incorporating a HCC. Hepatic progenitor cells,
the preparative mature hepatocytes or cholangiocytes, have been
identified in HA (12). As
previously noted, marrow-derived hepatic stem cells may transform
to both the atypical hepatocytes of HA and bone marrow
differentiated cells (9).
The DCP and AFP values are well-known tumor markers
of HCC. In the present case, AFP was within normal limits during
disease progression; however, DCP was markedly elevated,
consistently with increased tumor size, and it returned to normal
after surgery. The development of HA or its malignant
transformation in GSD-I patients is not necessarily associated with
the value of serum AFP (13,14), whereas elevation of the serum DCP
levels has been reported in a small number of patients with HA with
malignant transformation (15).
Therefore, the value of DCP may be useful for predicting the
malignant transformation of HA.
Malignant transformation of HA is relatively rare
and has been reported to be associated with dysregulation of
β-catenin (2,5,6), which
plays an important role in the Wnt signaling pathway (7). However, a previous study revealed that
neither nuclear accumulation of β-catenin detected by
immunohistochemistry, nor mutation in exon 3 of β-catenin detected
by direct sequencing, were observed in patients with HA, or HCC
with HA (3). In our case, nuclear
accumulation of β-catenin was observed on immunohistochemical
examination, but no mutation in exon 3 of β-catenin was detected in
HCC. The activation of the Wnt signaling pathway due to factors
other than mutation of β-catenin may have occurred during malignant
transformation in the present case. Therefore, malignant
transformation of HA is unlikely to follow the same pathway.
We herein report malignant transformation in a
patient with increased DCP levels, who underwent resection of HCC
arising from HA with bone marrow metaplasia producing three series
of hematopoietic cells, based on GSD-I. The increased size and
number of cells, as well as the elevated DCP levels, may be
considered as indicators of the malignant transformation of HA.
Glossary
Abbreviations
Abbreviations:
|
HA
|
hepatocellular adenoma
|
|
DCP
|
des-gamma-carboxy prothrombin
|
|
GSD
|
glycogen storage disease
|
|
AFP
|
α-fetoprotein
|
|
CT
|
computed tomography
|
References
|
1
|
Edmondson HA, Henderson B and Benton B:
Liver-cell adenomas associated with use of oral contraceptives. N
Engl J Med. 294:470–472. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rebouissou S, Bioulac-Sage P and
Zucman-Rossi J: Molecular pathogenesis of focal nodular hyperplasia
and hepatocellular adenoma. J Hepatol. 48:163–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Micchelli ST, Vivekanandan P, Boitnott JK,
Pawlik TM, Choti MA and Torbenson M: Malignant transformation of
hepatic adenomas. Mod Pathol. 21:491–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buell JF, Tranchart H, Cannon R and Dagher
I: Management of benign hepatic tumors. Surg Clin North Am.
90:719–735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agrawal S, Agarwal S, Arnason T, Saini S
and Belghiti J: Management of hepatocellular adenoma: Recent
advances. Clin Gastroenterol Hepatol. 13:1221–1230. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zucman-Rossi J, Jeannot E, Nhieu JT,
Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis
V, Michalak S, et al: Genotype-phenotype correlation in
hepatocellular adenoma: New classification and relationship with
HCC. Hepatology. 43:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Polakis P: The oncogenic activation of
beta-catenin. Curr Opin Genet Dev. 9:15–21. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moriura S, Kuroda M, Kimura A, Iwatsuka Y,
Ikeda S, Sakai T and Usui A: Case report: Hepatic adenoma with bone
marrow metaplasia in a patient with glycogen storage disease type
Ia. J Gastroenterol Hepatol. 11:556–559. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramacciato G, Nigri GR, Aurello P,
D'Angelo F, Pezzoli F, Rossi S, Pilozzi E, Ercolani G and Ravaioli
M: Giant hepatic adenoma with bone marrow metaplasia not associated
with oral contraceptive intake. World J Surg Oncol. 25:42006.
|
|
10
|
Ishak KG, Sharp HL and Schwarzenberg SJ:
Metabolic errors and liver diseaseMacSween RNM, Burt AD, Portmann
BC, Ishak KG, Scheuer PJ and Anthony PP: Pathology of the liver.
London: Churchill Livingstone; 2002, PubMed/NCBI
|
|
11
|
Gordon SC, Reddy KR, Livingstone AS,
Jeffers LJ and Schiff ER: Resolution of a
contraceptive-steroid-induced hepatic adenoma with subsequent
evolution into hepatocellular carcionoma. Ann Intern Med.
105:547–549. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Libbrecht L, De Vos R, Cassiman D, Desmet
V, Aerts R and Roskams T: Hepatic progenitor cells in
hepatocellular adenomas. Am J Surg Pathol. 25:1388–1396. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bianchi L: Glycogen storage disease I and
hepatocellular tumours. Eur J Pediatr. 152(Suppl 1): S63–S70. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee PJ: Glycogen storage disease type I:
Pathophysiology of liver adenomas. Eur J Pediatr. 161(Suppl 1):
S46–S49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito M, Sasaki M, Wen CY, Nakashima M, Ueki
T, Ishibashi H, Yano M, Kage M and Kojiro M: Liver cell adenoma
with malignant transformation: A case report. World J
Gastroenterol. 9:2379–2381. 2003. View Article : Google Scholar : PubMed/NCBI
|