Introduction
Gastric cancer is the second most common cause of
cancer-related mortality and the fourth most commonly diagnosed
type of cancer worldwide (1,2). The majority of gastric cancers are
diagnosed at an advanced or metastatic stage, with a 5-year
survival rate of 5–20% and a median overall survival of <1 year
(1). In countries with a high
gastric cancer prevalence, such as Japan, early screening programs
help to detect early-stage gastric cancer, which has a 5-year
survival rate of 90%. The three most common types of gastric
malignancies are gastric adenocarcinoma, gastrointestinal stromal
tumor and primary gastric lymphoma. The occurrence of synchronous
gastric adenocarcinoma and lymphoma is rare, and there is currently
no consensus regarding their management. Thus, decision-making
regarding the optimal treatment strategy may be challenging.
We herein describe the case of a patient who was
diagnosed with synchronous gastric adenocarcinoma and gastric
diffuse large B-cell lymphoma. Prioritizing treatment in such
patients is crucial, and certain factors, such as Helicobacter
pylori (H. pylori) infection, must be taken into
consideration during the decision-making process.
Case report
A 51-year-old Chinese man was referred to the
Taichung Veterans General Hospital from a community hospital with a
history of general malaise, poor appetite, abdominal fullness and a
weight loss of 10% over the past month. The patient was a chronic
hepatitis B carrier, had a 30 pack-year smoking history (one pack
per day for 30 years), and had suffered from epigastric discomfort
for several years without seeking medical attention. There was no
fever or night sweats. An abdominal contrast-enhanced computed
tomography scan revealed multiple lymphadenopathies in the
abdominal cavity, and the initial esophagogastroduodenoscopy
revealed an irregularly elevated area in the lower-to-middle
gastric body; the biopsies showed moderately differentiated
adenocarcinoma and diffuse mixed small and large B-cell lymphoma.
No H. pylori was identified on examination of Giemsa-stained
specimens. As the patient refused total gastrectomy, one cycle of
epirubicin, cisplatin and fluorouracil (ECF regimen) was initially
administered for gastric adenocarcinoma as neoadjuvant therapy; 1
month later, a bone marrow biopsy revealed diffuse mixed small and
large B-cell lymphoma negative for CD20 expression, and the patient
received 8 cycles of cyclophosphamide, adriamycin, vincristine and
prednisone (CHOP regimen).
Seven months later, the patient underwent distal
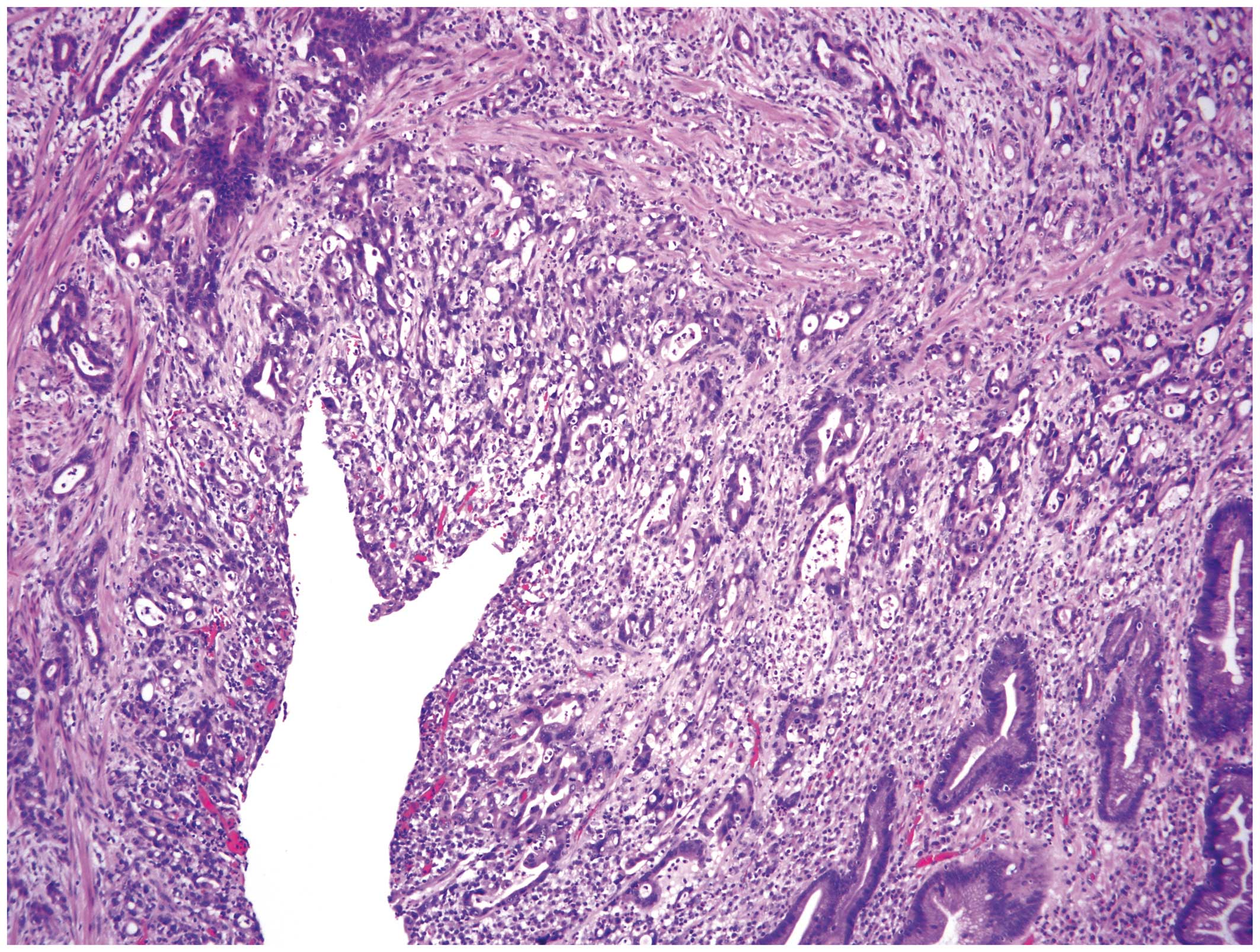
subtotal gastrectomy and the pathological examination confirmed the
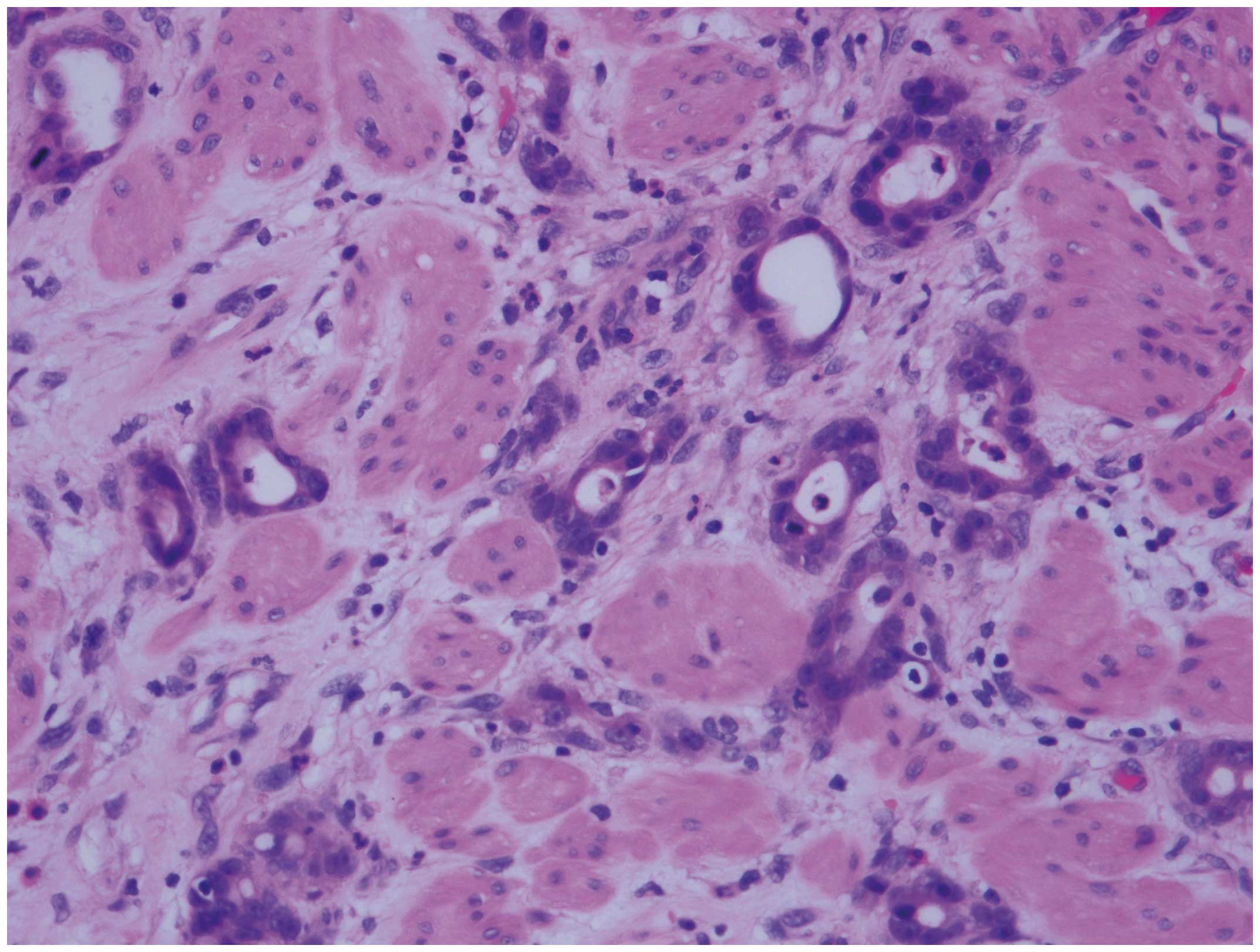
diagnosis of poorly differentiated adenocarcinoma (Fig. 1) and diffuse large B-cell lymphoma
(DLBCL) (Fig. 2), positive for CD20
and negative for H. pylori. Following surgery, the patient
received 8 cycles of rituximab, cyclophosphamide, mitoxantrone,
vincristine and prednisone (R-CNOP regimen) instead of CHOP to
reduce cardiotoxicity, and achieved complete remission of the
DLBCL. The patient declined further chemotherapy for the gastric
adenocarcinoma due to the deterioration of his liver function.
Three years later, the patient presented with
obstructive jaundice. Laparoscopic biopsy at the porta hepatis
showed moderately to poorly differentiated metastatic
adenocarcinoma (Fig. 3).
Immunohistochemical staining revealed 2+ human epidermal growth
factor-2 (HER-2) expression. Four cycles of capecitabine plus
oxaliplatin were administered and trastuzumab treatment was
recommended. However, trastuzumab was not initiated due to the
patient's financial difficulties. After 6 months, the patient
achieved near complete remission radiographically and was followed
up for another 6 months at the oncology clinic; however, he
succumbed to metastatic disease after ~1 year.
Discussion
Gastric cancer is the second most common cause of
cancer-related mortality worldwide (1–3).
Approximately 70% of gastric cancer cases are associated with H.
pylori infection, and 5.5% of all cancer cases globally are
H. pylori-associated gastric cancer (2,4,5). H. pylori infection is very
common in several parts of the world; its prevalence may be ≥50% in
certain areas, particularly in developing countries (4). Among individuals infected by H.
pylori, ~1–2% may develop gastric cancer, including
adenocarcinoma and mucosa-associated lymphoid tissue (MALT)
lymphoma (3,6,7). Risk
factors include the strain of H. pylori, the duration of the
infection, host genetic polymorphisms, and diet or other
environmental factors (8–13). In cases with synchronous
adenocarcinoma and lymphoma, H. pylori infection was present
in 92% of the cases in Eastern countries and 68% of the cases in
Western countries (14), although it
remains rare for synchronous tumors. Among all histological types
of gastric lymphoma, DLBCL and MALT lymphoma are the types most
significantly associated with H. pylori infection (15,16). The
possible mechanism underlying H. pylori as a causative
factor for gastric lymphoma is that chronic infection with H.
pylori causes hormonal and cellular changes and damages the
gastric cells. The damaged gastric cells then induce clonal
expansion of B cells (17,18). Thus, the current concept suggests
H. pylori eradication as an effective method in treating
low-grade gastric lymphoma (18,19). In
high-grade gastric lymphoma or DLBCL, due to a higher number of
genetic mutations, the response to H. pylori eradication
appears to be more limited (15,20).
The positive correlations between Epstein-Barr virus
(EBV) infection, gastric carcinoma and DLBCL have been investigated
and confirmed (21,22). Approximately 10% of gastric
carcinomas are EBV-positive, as are 9% of DLBCLs (21,22). The
positivity of EBV infection in patients with DLBCL is associated
with poorer response to treatment and survival (22). Chronic HBV infection may increase the
risk of non-Hodgkin lymphoma (NHL) by 3 times, particularly in
DLBCL (23,24).
Our patient was diagnosed with synchronous gastric
adenocarcinoma and diffuse large B-cell lymphoma, whereas H.
pylori infection was not identified. Since the patient
initially refused gastrectomy and was later found to have
co-existing DLBCL with bone marrow involvement, the CHOP regimen
was recommended prior to receiving subtotal gastrectomy, as DLBCL
is an aggressive NHL and bone marrow involvement indicates a more
aggressive condition with a worse outcome. Patients with chronic
hepatitis B infection may have compromised liver function and may
not be able to tolerate the stronger hepatotoxicity associated with
standard chemotherapy for gastric adenocarcinoma. Our patient
presented with metastasis of residual adenocarcinoma 3 years after
the gastrectomy. Although the patient responded well to standard
chemotherapy, from 2010 onwards there is another option for
patients with synchronous tumors and HER-2 positivity.
Studies suggest that the use of trastuzumab in
patients with HER2-positive advanced gastric or gastro-oesophageal
junction cancer significantly improved overall survival, with a 26%
reduction in mortality and prolongation of the median overall
survival (13.8 vs. 11.1 months) (1).
The addition of trastuzumab to chemotherapy does not increase the
toxicity associated with standard fluoropyrimidine-based
(5-fluorouracil) and platinum-based chemotherapy (1). Treatment with trastuzumab and platinum
may be a suitable and effective option for patients with
HER-2-positive gastric adenocarcinoma who cannot tolerate strong
hepatotoxicity. In the current 2016 NCCN guidelines for gastric
cancer treatment, the addition of trastuzumab to standard
chemotherapy is recommended as a first-line treatment option for
HER-2-positive patients (25). For
cases similar to our patient, trastuzumab may be added regardless
of the status of HER-2 expression, since the biopsy result may not
represent the expression status in all malignant tissues and tumor
cells exhibit a high mutation rate.
In conclusion, for patients diagnosed with
synchronous gastric adenocarcinoma and lymphoma, a number of
factors must be taken into consideration during decision-making in
terms of which cancer to treat first and which is the optimal
regimen. EBV and HBV play important roles in adenocarcinoma as well
as lymphoma. HER-2-positive patients with poor liver function may
be treated with trastuzumab in addition to platinum-based
chemotherapy. Surgical resection and subsequent pathological
examination of the tumor may offer more precise information
regarding the tumor types and optimal treatment.
Acknowledgements
The present study was performed in Taichung Veterans
General Hospital (Taichung, Taiwan, R.O.C.). The patient has signed
an informed consent regarding the publication of the case
details.
References
|
1
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim SS, Ruiz VE, Carroll JD and Moss SF:
Helicobacter pylori in the pathogenesis of gastric cancer and
gastric lymphoma. Cancer Lett. 305:228–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Correa P and Piazuelo MB: Helicobacter
pylori infection and gastric adenocarcinoma. US Gastroenterol
Hepatol Rev. 7:59–64. 2011.PubMed/NCBI
|
|
4
|
Testerman TL and Morris J: Beyond the
stomach: An updated view of Helicobacter pylori pathogenesis,
diagnosis, and treatment. World J Gastroenterol. 20:12781–12808.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mbulaiteye SM, Hisada M and El-Omar EM:
Helicobacter pylori associated global gastric cancer burden. Front
Biosci (Landmark Ed). 14:1490–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herrera V and Parsonnet J: Helicobacter
pylori and gastric adenocarcinoma. Clin Microbiol Infect.
15:971–976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brawner KM, Morrow CD and Smith PD:
Gastric microbiome and gastric cancer. Cancer J. 20:211–216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atherton JC, Cao P, Peek RM Jr, Tummuru
MK, Blaser MJ and Cover TL: Mosaicism in vacuolating cytotoxin
alleles of Helicobacter pylori. Association of specific vacA types
with cytotoxin production and peptic ulceration. J Biol Chem.
270:17771–17777. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ziel KA, Campbell CC, Wilson GL and
Gillespie MN: Ref-1/Ape is critical for formation of the
hypoxia-inducible transcriptional complex on the hypoxic response
element of the rat pulmonary artery endothelial cell VEGF gene.
FASEB J. 18:986–988. 2004.PubMed/NCBI
|
|
10
|
Harris PR, Smythies LE, Smith PD and
Perez-Perez GI: Role of childhood infection in the sequelae of H.
pylori disease. Gut Microbes. 4:426–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee WP, Tai DI, Lan KH, Li AF, Hsu HC, Lin
EJ, Lin YP, Sheu ML, Li CP, Chang FY, et al: The −251T allele of
the interleukin-8 promoter is associated with increased risk of
gastric carcinoma featuring diffuse-type histopathology in Chinese
population. Clin Cancer Res. 11:6431–6441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shikata K, Kiyohara Y, Kubo M, Yonemoto K,
Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y, et al:
A prospective study of dietary salt intake and gastric cancer
incidence in a defined Japanese population: The Hisayama study. Int
J Cancer. 119:196–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Correa P, Fontham ET, Bravo JC, Bravo LE,
Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD and Mera
R: Chemoprevention of gastric dysplasia: Randomized trial of
antioxidant supplements and anti-Helicobacter pylori therapy. J
Natl Cancer Inst. 92:1881–1888. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan AO, Chu KM, Yuen ST, Leung SY, Lam SK
and Wong J: Synchronous gastric adenocarcinoma and
mucosa-associated lymphoid tissue lymphoma in association with
Helicobacter pylori infection: Comparing reported cases between the
East and West. Am J Gastroenterol. 96:1922–1924. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Sanjose S, Dickie A, Alvaro T, Romagosa
V, Villanueva M Garcia, Domingo-Domenech E, de Sevilla A Fernandez
and El-Omar E: Helicobacter pylori and malignant lymphoma in Spain.
Cancer Epidemiol Biomarkers Prev. 13:944–948. 2004.PubMed/NCBI
|
|
16
|
Parsonnet J, Hansen S, Rodriguez L, Gelb
AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH and Friedman GD:
Helicobacter pylori infection and gastric lymphoma. N Engl J Med.
330:1267–1271. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hussell T, Isaacson PG, Crabtree JE, Dogan
A and Spencer J: Immunoglobulin specificity of low grade B cell
gastrointestinal lymphoma of mucosa-associated lymphoid tissue
(MALT) type. Am J Pathol. 142:285–292. 1993.PubMed/NCBI
|
|
18
|
Wotherspoon AC, Doglioni C, Diss TC, Pan
L, Moschini A, de Boni M and Isaacson PG: Regression of primary
low-grade B-cell gastric lymphoma of mucosa-associated lymphoid
tissue type after eradication of Helicobacter pylori. Lancet.
342:575–577. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cavanna L, Pagani R, Seghini P, Zangrandi
A and Paties C: High grade B-cell gastric lymphoma with complete
pathologic remission after eradication of Helicobacter pylori
infection: Report of a case and review of the literature. World J
Surg Oncol. 6:352008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Ye H, Ruskone-Fourmestraux A, De
Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, Wündisch
T, et al: T(11;18) is a marker for all stage gastric MALT lymphomas
that will not respond to H. pylori eradication. Gastroenterology.
122:1286–1294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukayama M and Ushiku T: Epstein-Barr
virus-associated gastric carcinoma. Pathol Res Pract. 207:529–537.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park S, Lee J, Ko YH, Han A, Jun HJ, Lee
SC, Hwang IG, Park YH, Ahn JS, Jung CW, et al: The impact of
Epstein-Barr virus status on clinical outcome in diffuse large
B-cell lymphoma. Blood. 110:972–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Engels EA, Cho ER and Jee SH: Hepatitis B
virus infection and risk of non-Hodgkin lymphoma in South Korea: A
cohort study. Lancet Oncol. 11:827–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yood M Ulcickas, Quesenberry CP Jr, Guo D,
Caldwell C, Wells K, Shan J, Sanders L, Skovron ML, Iloeje U and
Manos MM: Incidence of non-Hodgkin's lymphoma among individuals
with chronic hepatitis B virus infection. Hepatology. 46:107–112.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
NCCN, . National Comprehensive Cancer
Network Guidelines for gastric cancer. Version 3. 2016.https://www.nccn.org/professionals/physician_gls/f_guidelines.aspAccessed
September 12, 2016.
|