Introduction
Carcinoma of the lower uterine segment (LUS) is a
malignant uterine tumor arising between the anatomical internal os
and histological internal os of the uterus. It is a rare tumor that
accounts for 3–3.5% of uterine corpus carcinomas (1). Carcinoma of the LUS is characterized by
deeper myometrial invasion and multiple lymph node metastases
compared with endometrial carcinoma, and a previous study indicated
an association with Lynch syndrome (1). A cancer that is widely present in the
whole body through the endocervix is excluded from the definition
of carcinoma of the LUS. It is often difficult to distinguish
between cervical cancer and uterine corpus cancer; however, the
treatment policies are different. Magnetic resonance imaging (MRI)
may be able to discriminate between the two conditions (2); other reports suggest that MRI is not
necessarily useful in this respect (3). Discrimination may also be possible by
immunohistochemical staining (4) or
detection of human papillomavirus (HPV) DNA (5); however, there is no established method
for this purpose.
Clear cell carcinoma of the uterine corpus is a rare
tumor that accounts for 1–6% of uterine corpus carcinomas. Clear
cell carcinoma is considered to be a type II tumor that is less
associated with estrogen and differs from endometrioid
adenocarcinoma (1). Following
surgical staging, patients with clear cell carcinoma are often
upstaged to a higher clinical stage compared with that found in
pre-surgical staging. Clear cell carcinoma is also less sensitive
to chemotherapy and radiotherapy compared with lower grade
endometrioid adenocarcinoma (6).
Therefore, the 5-year overall survival rate of clear cell carcinoma
of the uterine corpus is only ~40%, which is markedly worse
compared with that of endometrioid adenocarcinoma (6). The present report described a rare case
of clear cell carcinoma arising from the LUS and is presented with
written informed consent from the patient.
Case report
The patient was a 50-year-old woman (gravida 2, para
2) with no other relevant medical and family history. She visited a
local clinic due to irregular vaginal bleeding for several months.
She was diagnosed with a uterine tumor and referred to Tachikawa
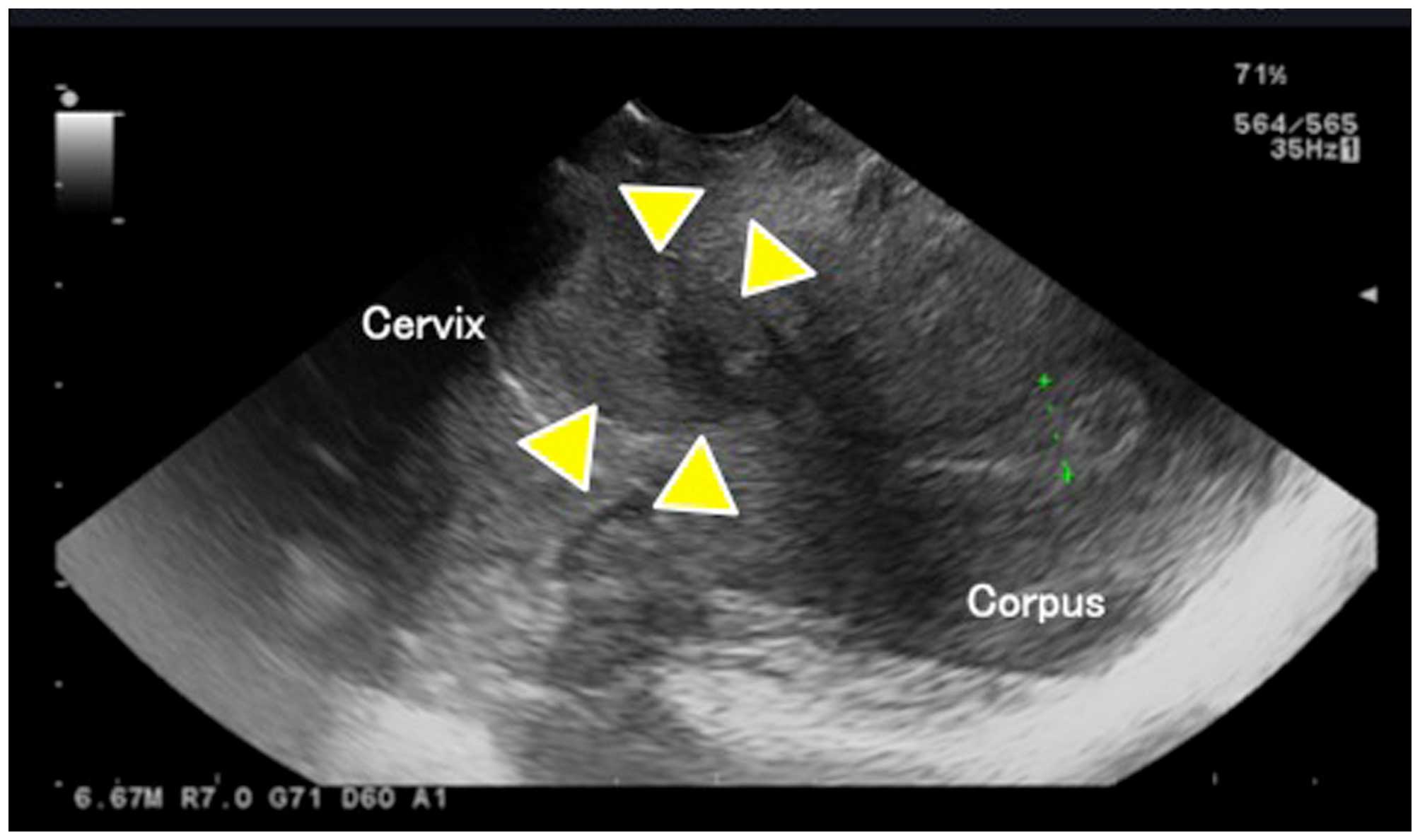
Hospital (Tokyo, Japan). Transvaginal ultrasound revealed a uterine
tumor of ~3 cm from the lower region of the uterine body through
the upper region of the cervix (Fig.
1). Serum carbohydrate antigen (CA)-125 was elevated to 111.0
U/ml (normal, <35 U/ml). Other tumor markers (SCC, CA19-9 and
CEA) were within normal ranges and other biochemical parameters in
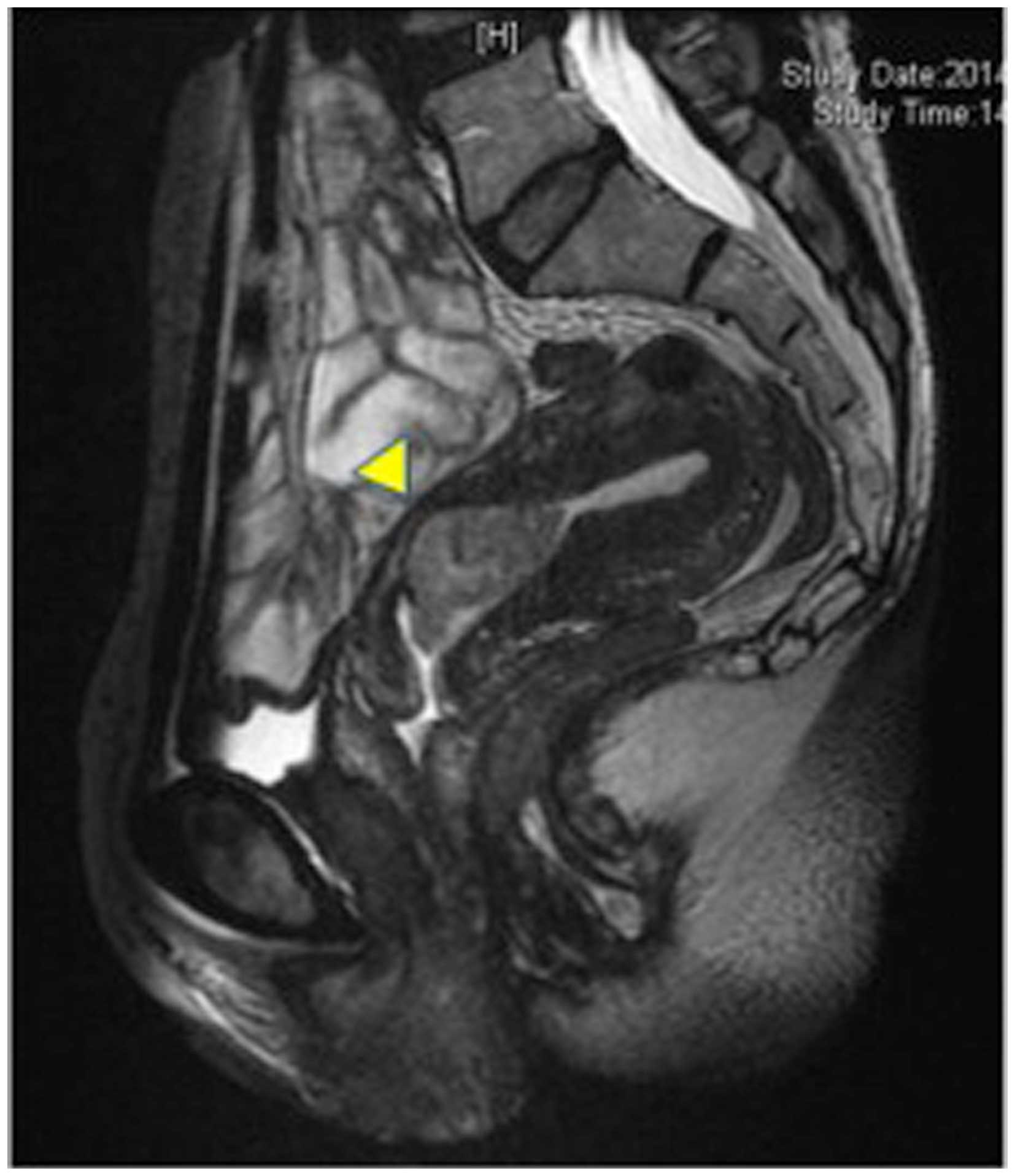
the blood were normal. MRI revealed the presence of a tumor mass
protruding into the uterine cavity from the lower portion of the
uterine body to the upper uterine cervix (Fig. 2). A cervical pap smear was negative
for intraepithelial lesions. An endocervical smear revealed
adenocarcinoma. A curettage biopsy of the uterine cervix revealed
atypical cells with atypical glands, and papillary formations and
clear cytoplasm, leading to diagnosis of clear cell carcinoma. An
endometrial biopsy was negative. Computed tomography revealed no
obvious lymph node metastasis or distant metastasis.
Based on a preoperative diagnosis of clear cell
carcinoma of the LUS, radical hysterectomy was performed with
bilateral salpingo-oophorectomy, pelvic lymph node dissection,
paraaortic lymph node dissection and omentectomy. Macroscopically,
a tumor of 4.0×5.5 cm was observed focally in the LUS, with no
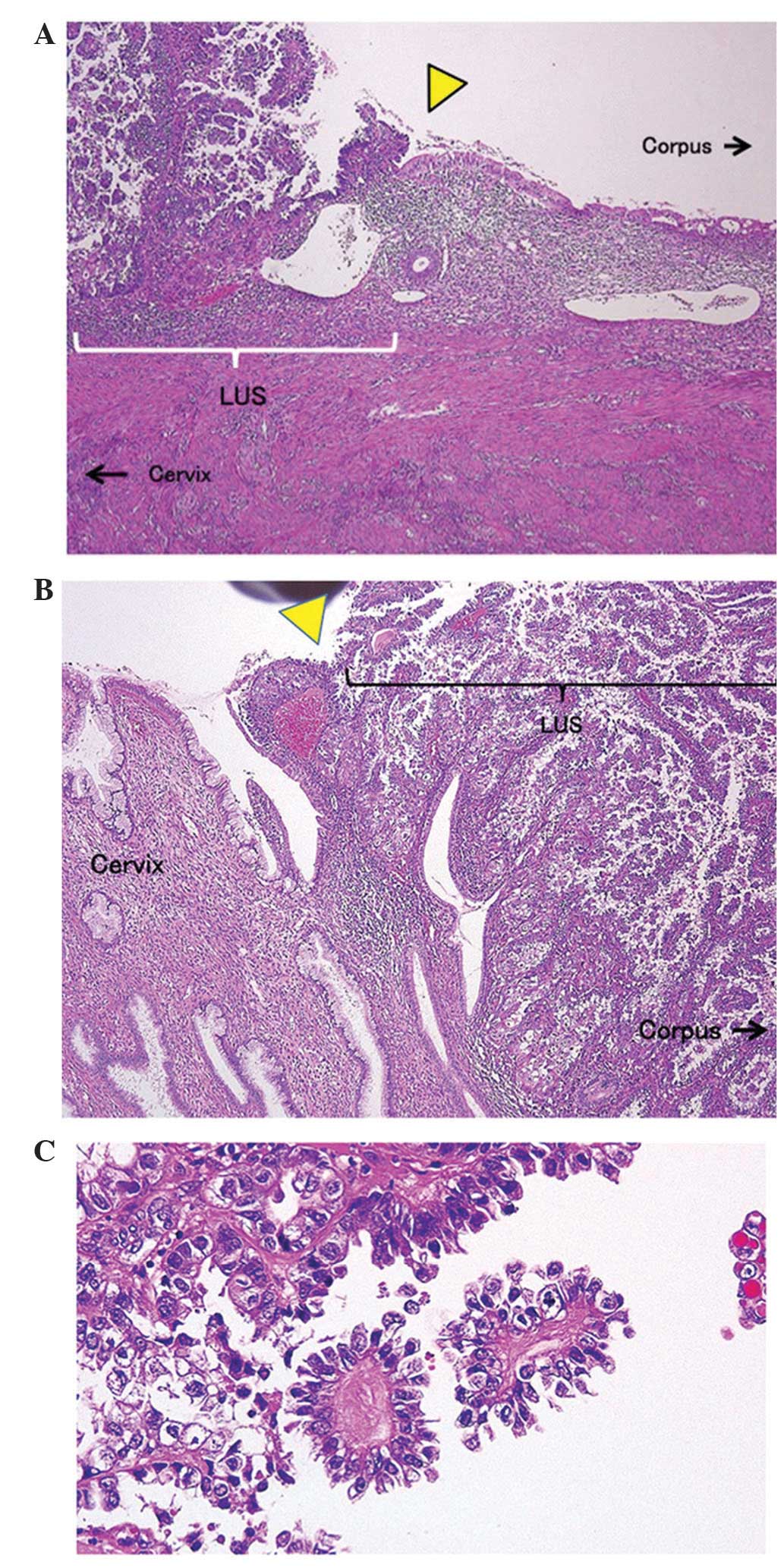
tumors in the uterine corpus and cervix (Fig. 3). No abnormal histopathological
findings were observed in the endometrium and uterine cervix
epithelium, but malignant cells with well-defined borders with the
uterine cervix epithelium and endometrial epithelium (front
formation) was observed in the LUS (Fig.
4A and B). The malignant cells exhibited tubular and papillary
formations. Additionally, hobnail cells with large nuclei,
chromatin condensation and high-grade dyskaryosis, and abundant
clear cytoplasms were observed. Based on these findings, the tumor
was diagnosed as clear cell carcinoma (Fig. 4C). Cervical stromal invasion was
marginally identified; however, no metastasis into the adnexa or
lymph nodes was observed. Finally, the tumor was diagnosed as
endometrial clear cell carcinoma arising from the LUS (FIGO stage
II). Adjuvant chemotherapy was performed. At the 1-year follow-up,
the patient was disease-free without local recurrence or
metastasis.
Discussion
Carcinoma of the LUS is managed as a cancer of the
uterine corpus; however, the characteristics differ from those of
other cancer types of the uterine corpus. The age of onset of
carcinoma of the LUS is younger (3,7) and the
tumor lacks type I characteristics, which include irregular
menstruation, nulliparity, infertility and a high frequency of
polycystic ovary syndrome (7).
Carcinoma of the LUS has also recently been associated with Lynch
syndrome. Westin et al (3)
found that 10/35 cases (29%) of carcinoma of the LUS also had Lynch
syndrome and that the hMSH2 mutation was present at a high
frequency. Hachisuga et al (7) reported that carcinoma of the LUS was
more frequently adenosquamous carcinoma with grade 3 pathological
features (7), whereas Watanabe et
al (8) and Westin et al
(3) suggested that no difference in
the histological type and grading existed between carcinoma of the
LUS and cancer of the uterine corpus. Deeper muscular invasion of
carcinoma of the LUS was observed compared with that of cancer of
the uterine corpus (3,7). In addition, the frequency of
metastasis-positive lymph node cytology in ascites, lymph and blood
vessels is higher in carcinoma of the LUS (3,8). These
characteristics are suggested to be due to the endometrium of the
LUS being thinner compared with that of the uterine corpus.
It is often difficult to determine whether a tumor
originates from the LUS or cervix, though the treatment differs for
the different tumor types. Haider et al (2) found that MRI is useful for
discriminating between endometrial cancer with cervical invasion
and cervical cancer, with a positive predictive value of 92% and a
negative predictive value of 88% for discriminating between these
conditions (2). By contrast, Westin
et al (3) found that MRI was
not necessarily useful for discrimination based on the inability to
diagnose the cancer origin in 56% of cases with uncertain primary
lesions, in which 23% of patients originally diagnosed with
cervical cancer were finally diagnosed with cancer of the LUS
(3). Immunohistochemical analysis is
another approach for discrimination. Typical cases of endometrial
cancer are positive for estrogen receptor (ER) and vimentin, and
negative for carcinoembryonic antigen (CEA), whereas cervical
cancer exhibits the opposite pattern. Therefore, it has been
suggested that a combination of markers may allow discrimination of
cervical cancer from endometrial cancer (4,9).
Detection of HPV DNA and immunostaining for p16 may also be useful
for discrimination of these conditions (5).
In the present case, discrimination was relatively
easy preoperatively since the tumor was limited to the LUS. In
general, pathological features of both the uterine endocervix and
endometrium are observed in the LUS epithelium and interstitial
tissue. However, the pathological findings in isolated specimens in
this case revealed that epithelia of the uterine cervix and corpus
were normal, and identified malignant cells with well-defined
borders within these epithelia. Clear cell carcinoma originating
from the LUS was definitively diagnosed histopathologically.
Clear cell carcinoma exhibits different
characteristics compared with endometrial cancer. Clear cell
carcinoma of the uterine corpus accounts for 1–6% of uterine corpus
carcinomas (6), and is considered to
be a poorly differentiated carcinoma, such as serous
adenocarcinoma. These tumors are classified as type II uterine
corpus cancer and are less associated with estrogen. Clear cell
carcinoma has a high propensity toward extrauterine spread and a
poor prognosis, with a previous study finding that 39% of patients
with clinical stage I or II clear cell carcinoma were upstaged to
III or IV, compared with 12% with an endometrioid subtype (6). Surgical treatment and adjuvant therapy
are used; however, there is limited evidence for the efficacy of
chemotherapy and radiation therapy.
To the best of our knowledge, this is the first
report of clear cell carcinoma of the LUS. The tumor exhibited the
characteristics of cancer of the LUS and of clear cell carcinoma. A
search for ‘clear cell’ and ‘lower uterine segment’ on Pubmed
resulted in no reports of clear cell carcinoma of the LUS.
Carcinoma of the LUS itself is relatively rare, and therefore, a
tumor of the LUS with clear cell carcinoma is particularly unusual.
Cancer of the LUS and uterine clear cell carcinoma exhibit
characteristics of type II uterine corpus cancer and may have
common risk factors. The present patient had delivered two children
and did not exhibit irregular menstruation or other risk factors
for type I uterine corpus cancer. This background is consistent
with the characteristics of cancer of the LUS; however, muscle
invasion only at the endocervix, a negative cytological examination
in ascitic fluid, lack of vascular invasion, and negative lymph
node metastasis are inconsistent with a tumor of the LUS.
Therefore, more cases are required to clarify the pathology of
clear cell carcinoma of the LUS.
References
|
1
|
Masuda K, Banno K, Yanokura M, Kobayashi
Y, Kisu I, Ueki A, Ono A, Nomura H, Hirasawa A, Susumu N, et al:
Carcinoma of the lower uterine segment (LUS): Clinicopathological
characteristics and association with Lynch Syndrome. Curr Genomics.
12:25–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haider MA, Patlas M, Jhaveri K, Chapman W,
Fyles A and Rosen B: Adenocarcinoma involving the uterine cervix:
Magnetic resonance imaging findings in tumours of endometrial,
compared with cervical, origin. Can Assoc Radiol J. 57:43–48.
2006.PubMed/NCBI
|
|
3
|
Westin SN, Lacour RA, Urbauer DL, Luthra
R, Bodurka DC, Lu KH and Broaddus RR: Carcinoma of the lower
uterine segment: A newly described association with Lynch syndrome.
J Clin Oncol. 26:5965–5971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCluggage WG, Sumathi VP, McBride HA and
Patterson A: A panel of immunohistochemical stains, including
carcinoembryonic antigen, vimentin, and estrogen receptor, aids the
distinction between primary endometrial and endocervical
adenocarcinomas. Int J Gynecol Pathol. 21:11–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ansari-Lari MA, Staebler A, Zaino RJ, Shah
KV and Ronnett BM: Distinction of endocervical and endometrial
adenocarcinomas: Immunohistochemical p16 expression correlated with
human papillomavirus (HPV) DNA detection. Am J Surg Pathol.
28:160–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hasegawa K, Nagao S, Yasuda M, Millan D,
Viswanathan AN, Glasspool RM, Devouassoux-Shisheboran M, Covens A,
Lorusso D, Kurzeder C, et al: Gynecologic Cancer InterGroup (GCIG)
consensus review for clear cell carcinoma of the uterine corpus and
cervix. Int J Gynecol Cancer. 24:(Suppl 3). S90–S95. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hachisuga T, Fukuda K, Iwasaka T, Hirakawa
T, Kawarabayashi T and Tsuneyoshi M: Endometrioid adenocarcinomas
of the uterine corpus in women younger than 50 years of age can be
divided into two distinct clinical and pathologic entities based on
anatomic location. Cancer. 92:2578–2584. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe Y, Nakajima H, Nozaki K, Ueda H,
Obata K, Hoshiai H and Noda K: Clinicopathologic and
immunohistochemical features and microsatellite status of
endometrial cancer of the uterine isthmus. Int J Gynecol Pathol.
20:368–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liao CL, Hsu JD, Lee MY, Kok LF, Li YJ,
Wang PH, Yao CC and Han CP: Distinguishing between primary
endocervical and endometrial adenocarcinomas: Is a 2-marker
(Vim/CEA) panel enough? Virchows Arch. 456:377–386. 2010.
View Article : Google Scholar : PubMed/NCBI
|