Introduction
Intravascular papillary endothelial hyperplasia
(IPEH), also referred to as intravascular angiomatosis, vegetant
intravascular hemangioendothelioma, Masson's pseudoangiosarcoma or
Masson's tumor, is a relatively uncommon vascular lesion caused by
extensive proliferation of endothelial cells (1–3). IPEH
was first described in 1923 by Pierre Masson as an intravascular
papillary proliferation formed within the lumen of an inflamed
hemorrhoidal plexus in a male patient, and termed
‘Hèmangioendothèliome vègètant intravasculaire’ (4). IPEH comprises ~2% of the vascular
tumors of the skin and subcutaneous tissue and it has a
predilection for the head, neck, trunk and the extremities
(5–7). The majority of the described cases in
the head and neck were reported in the oral mucosa, lower and upper
lips, tongue, gingiva, skin, subcutaneous tissue of the face and
scalp, intracranium, orbit and ocular adnexa. Clinically, IPEH may
simulate mucocele, hemangioma, lymphangioma, angiosarcoma,
hematoma, Kaposi's sarcoma, hemangioendothelioma, thrombosed vein,
phlebectasia, traumatic fibroma, melanoma, fibroepithelial polyp,
non-odontogenic soft tissue infection, intramasseteric abscess,
cysticercosis, benign neoplasms of smooth muscle origin, and
reactive and neoplastic neural lesions, such as traumatic neuroma,
neurofibroma and neurilemmoma (8–10).
Radiologically, no specific findings have been found to be
characteristic of IPEH.
The microscopic examination of IPEH is pathognomonic
and immunohistochemical evaluation is not generally required for
diagnosis (11). Useful points in
the differential diagnosis are the lack of cellular anaplasia,
necrosis, mitotic activity and nuclear atypia; most of the
papillary structures are associated with thrombi, and the papillae
are usually covered by no more than two endothelial cell layers.
Additionally, the proliferative process occurs exclusively in the
intravascular space (11). Hashimoto
et al (12) classified IPEH
into three types as follows: Type I (pure form), is the most common
subtype and is characterized by dilated vascular spaces; type II
(mixed form) occurs in pre-existing varices, capillary or cavernous
hemangiomas, lymphangiomas, pyogenic granulomas, arteriovenous
malformations and blue rubber bleb nevi; type III (undermined type)
is the least common variant and is characterized by an
extravascular location, developing in the bed of a hematoma,
frequently associated with trauma.
Immunohistochemically, IPEH lesions are positive for
CD31, CD34, vimentin, α-smooth muscle actin, factor VIII, XIIIa,
type IV collagen, CD105, Ki-67 and ferritin (13,14).
CD31 and CD34 are considered to be the most sensitive markers
indicating the vascular origin of the lesion, while staining for
the other vascular markers may be variable. Ferritin is a
ubiquitous protein involved in intracellular iron metabolism, due
to its ability to sequester free iron in a non-toxic and
bioavailable form (15). Two
functionally and genetically distinct ferritin subunits exist:
L-ferritin and H-ferritin, also referred to as light-chain and
heavy-chain ferritin (FLC and FHC, respectively). A single ferritin
complex may include both H and L subunits and the H:L ratio within
a single complex may modulate its functional potential in a
context-dependent manner. In addition to its intracellular form,
ferritin is also an abundant protein in the circulation, as well as
in the synovial and cerebrospinal fluids (16). Serum ferritin is associated with a
higher risk for certain cancers, and higher levels are detected in
several malignancies, including Hodgkin's lymphoma, neuroblastoma,
lung, ovarian, pancreatic, intestinal, hepatic, gastric and breast
cancers (17). The level of serum
ferritin may also be modified by inflammation. Indeed, inflammatory
cytokines may regulate the expression of ferritin on two levels: A
transcriptional level (mainly H-ferritin) and a translational level
(both H- and L-ferritin) (18). In
addition, a number of authors suggested a critical role of ferritin
in the regulation of angiogenesis, exerting a cytoprotective effect
on endothelial cells (19). We
herein present a new case of Masson's tumor in the parotid gland,
which, to the best of our knowledge, is the second reported in the
international literature.
Case report
A 70-year-old woman was admitted to the Unit of Oral
and Maxillofacial Surgery, with a 5-year duration of a right
parotid painless enlargement, causing facial asymmetry. Magnetic
resonance imaging (MRI) and computed tomography-based
three-dimensional (3D-CT) templating were used for preoperative
planning.
The patient appeared to be in overall good health.
On clinical examination, a lesion sized 2×1 cm was identified in
the right parotid gland. The lesion was freely movable, tender on
digital palpation, non-fluctuant, rubbery in consistency, with
well-defined margins, not fixed to the underlying or overlying
structures. There were no signs of neurological abnormalities, such
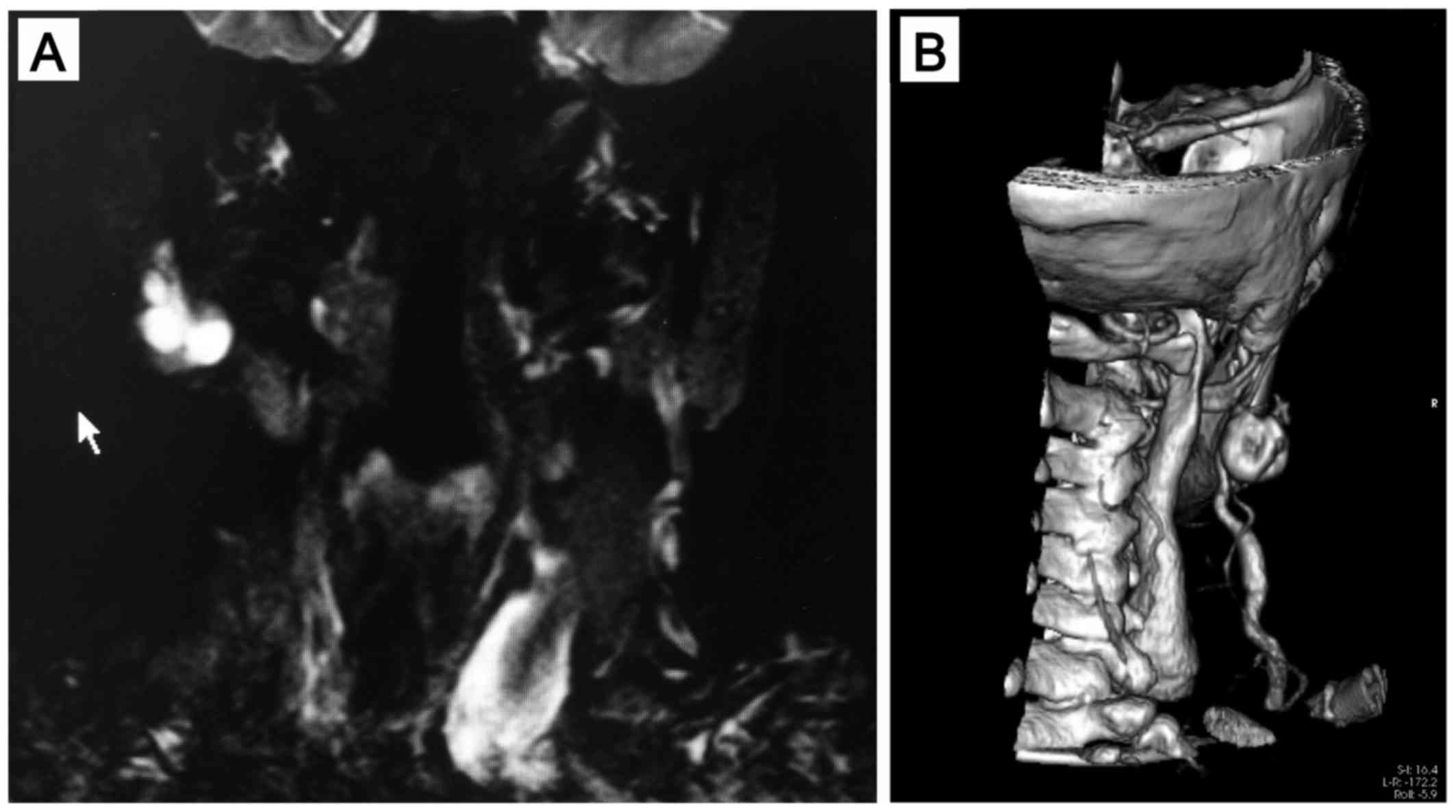
as facial paralysis. MRI revealed an irregular lesion with smooth
margins, appearing isointense on T1 sequences, heterogeneously
hyperintense on long TR sequences with homogeneous contrast
enhancement, initially considered to be compatible with pleomorphic
adenoma (Fig. 1A).
3D-CT-reconstructed images revealed an association of the lesion
with the vessels (Fig. 1B).
Extracapsular dissection of the lesion was performed under general
anesthesia (20). On macroscopic
examination, the resected specimen was a tender and brownish-red
nodule, sized 2×1 cm.
Histological examination revealed a hemorrhagic
area, forming an organized thrombus with areas of intravascular
papillary formation, covered by flat to plump endothelial cells. No
necrosis, mitoses or significant pleomorphism were identified
(Fig. 2A and B). The morphological
diagnosis was IPEH, type III variant. Immunohistochemical analysis
revealed cell positivity for FLC, as well as FHC, vimentin and CD31
(Fig. 2C-F).
Discussion
The pathogenesis of IPEH remains unclear. Masson
described IPEH as the result of endothelial cells proliferating
into the vessel lumen, followed by obstruction and a secondary red
infarct; in this description, Masson considered IPEH as a neoplasm
(4). On the basis of IPEH incidence,
with a male:female ratio of 1:1.3, a hormonal role has also been
suggested (9). Subsequently, IPEH
was defined as an uncommon benign, non-neoplastic vascular lesion,
consisting of endothelial cell proliferation caused by a reactive
process (21,22). In this context, significant attention
has been directed to the association of IPEH with thrombus
formation, with several hypotheses on the potential causative role
in the thrombotic process. Levere et al reported elevated
level of basic fibroblast growth factor (bFGF) in cases of IPEH
when compared with non-IPEH organizing thrombi; in addition, they
suggested that bFGF was released by macrophages in response to
trauma (23). A number of studies
pointed to IPEH as an unusual form of thrombus that undergoes
fragmentation, followed by active endothelization of its fragments
(24). Accordingly, Albrecht et
al described the positivity of thrombus-lining cells for
ferritin, vimentin and factor VIII-related antigen, indicating
cells progressing from a histiocytic to an endothelial phenotype
(25). In particular, ferritin
positivity was reported in the earlier stages of lesion
development, while vimentin and factor VIII-related antigen
positivity have been reported in the final stage (5,26).
Surprisingly, in the present case, the lesion exhibited
simultaneous expression for vimentin, CD31 and both ferritin
subunits. From the more recent literature, it appears that ferritin
may modulate the balance between pro- and anti-angiogenic factors.
In particular, Tesfay et al demonstrated that ferritin was
able to block the anti-angiogenic activity of two-chain
high-molecular-weight kininogen (HKa), interfering with the
anti-adhesive and anti-proliferative signaling of HKa. Furthemore,
both FHC and FLC were able to counteract the inhibitory effects of
HKa on the proliferation, adhesion, migration and viability of
endothelial cells. The regulatory role of ferritin on angiogenesis
has been confirmed by other studies, which suggest it to be
primarily due to FLC (27).
Accordingly, in our study, the intensity of FLC staining appeared
to be higher compared with that of FHC staining. In conclusion, we
herein report a rare case of Masson's tumor of the parotid gland,
the second case described in literature to date. The
immunohistochemical findings suggest the potential role of
ferritin, not only in the pathogenesis, but also in the maintenance
of IPEH. However, further investigations are required in order to
elucidate the precise role of ferritin in cell progression from a
histiocytic to an endothelial phenotype.
References
|
1
|
Luce EB, Montgomery MT, Redding SW and
Aufdemorte TB: Intravascular angiomatosis (Masson's lesion). J Oral
Maxillofac Surg. 46:736–741. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuo T, Sayers CP and Rosai J: Masson's
‘vegetant intravascular hemangioendothelioma’: a lesion often
mistaken for angiosarcoma: study of seventeen cases located in the
skin and soft tissues. Cancer. 38:1227–1236. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Escasany R Torne and Millet P Umbert:
Masson's pseudoangiosarcoma of the tongue: Report of two cases. J
Cutan Pathol. 12:66–71. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masson P: Hèmangioendothèliome vègètant
intravasculaire. Bull Soc Anat Paris. 93:517–523. 1923.
|
|
5
|
Tosios K, Koutlas IG and Papanicolaou SI:
Intravascular papillary endothelial hyperplasia of the oral soft
tissues: Report of 18 cases and review of the literature. J Oral
Maxillofac Surg. 52:1263–1268. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hutcheson EL, Picarella EA and Blevins PK:
Masson's tumor of the hand: a case report and brief literature
review. Ann is Plast Surg. 69:338–339. 2012. View Article : Google Scholar
|
|
7
|
Tedla M, Bežová M, Biró C, Tedlová E, Eng
CY and Zeleník K: Intravascular papillary endothelial hyperplasia
of larynx: Case report and literature review of all head and neck
cases. Otolaryngol Pol. 68:200–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moon WS, Chung GH and Hong KH:
Intravascular papillary endothelial hyperplasia in a vascular
lesion of the paranasal sinus. Arch Pathol Lab Med. 124:1224–1227.
2000.PubMed/NCBI
|
|
9
|
Makos CP and Nikolaidou AJ: Intravascular
papillary endothelial hyperplasia (Masson's tumor) of oral mucosa.
Presentation of two cases and review. Oral Oncol Extra. 40:59–62.
2004. View Article : Google Scholar
|
|
10
|
Murugaraj V, Kingston GT, Patel M and
Anand R: Intravascular papillary endothelial hyperplasia (Masson's
tumour) of the oral mucosa. Br J Oral Maxillofac Surg. 48:e16–e17.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sarode GS, Sarode SC and Karmarkar SP:
Oral intravascular papillary endothelial hyperplasia (Masson's
tumor): A review of literature. J Oral Maxillofac Surg Med Pathol.
26:73–79. 2014. View Article : Google Scholar
|
|
12
|
Hashimoto H, Daimaru Y and Enjoji M:
Intravascular papillary endothelial hyperplasia. A
clinicopathologic study of 91 cases. Am J Dermatopathol. Dec;5(6):
539–546. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akdur NC, Donmez M, Gozel S, Ustun H and
Hucumenoglu S: Intravascular papillary endothelial hyperplasia:
Histomorphological and immunohistochemical features. Diagn Pathol.
8:1672013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guledgud MV, Patil K, Saikrishna D,
Madhavan A and Yelamali T: Intravascular papillary endothelial
hyperplasia: Diagnostic sequence and literature review of an
orofacial lesion. Case Rep Dent. 2014:9345932014.PubMed/NCBI
|
|
15
|
Zolea F, Biamonte F, Candeloro P, Di Sanzo
M, Cozzi A, Di Vito A, Quaresima B, Lobello N, Trecroci F, Di
Fabrizio E, et al: H ferritin silencing induces protein misfolding
in K562 cells: A Raman analysis. Free Radic Biol Med. 89:614–623.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meyron-Holtz EG, MosheBelizowski S and
Cohen LA: A possible role for secreted ferritin in tissue iron
distribution. J Neural Transm Vienna. 118:337–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alkhateeb AA and Connor JR: The
significance of ferritin in cancer: Anti-oxidation, inflammation
and tumorigenesis. Biochim Biophys Acta. 1836:245–254.
2013.PubMed/NCBI
|
|
18
|
Rogers JT, Bridges KR, Durmowicz GP, Glass
J, Auron PE and Munro HN: Translational control during the acute
phase response. Ferritin synthesis in response to interleukin-1. J
Biol Chem. 265:14572–14578. 1990.PubMed/NCBI
|
|
19
|
Tesfay L, Huhn AJ, Hatcher H, Torti FM,
Torti SV and Ahmir A: Ferritin blocks inhibitory effects of
two-chain high molecular weight kininogen (HKa) on adhesion and
survival signaling in endothelial cells. PLoS One. 7:e400302012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cristofaro MG, Allegra E, Giudice A,
Colangeli W, Caruso D, Barca I and Giudice M: Pleomorphic adenoma
of the parotid: Extracapsular dissection compared with superficial
parotidectomy-a 10-year retrospective cohort study. Sci World J.
2014:5640532014. View Article : Google Scholar
|
|
21
|
Henschen F: L'endovasculite proliférante
thrombopoïétique dans la lesion vasculaire locale. Ann Anat Path.
9:113–121. 1932.
|
|
22
|
Csank GA and Curletti EL: Intravascular
papillary endothelial hyperplasia: Challenge in diagnosis and
vascular reconstruction-a case report. Vasc Endovascular Surg.
32:295–299. 1998. View Article : Google Scholar
|
|
23
|
Levere SM, Barsky SH and Meals RA:
Intravascular papillary endothelial hyperplasia: A neoplastic
‘actor’ representing an exaggerated attempt at recanalization
mediated by basic fibroblast growth factor. J Hand Surg Am.
19:559–564. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buchner A, Merrell PW, Carpenter WM and
Leider AS: Oral intravascular papillary endothelial hyperplasia. J
Oral Pathol Med. 19:419–422. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Albrecht S and Kahn HJ:
Immunohistochemistry of intravascular papillary endothelial
hyperplasia. J Cutan Pathol. 17:16–21. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katzman B, Caligiuri DA, Klein DM,
Nicastri AD and Chen P: Recurrent intravascular papillary
endothelial hyperplasia. J Hand Surg [Br]. 22:113–115. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coffman LG, Parsonage D, D'Agostino R Jr,
Torti FM and Torti SV: Regulatory effects of ferritin on
angiogenesis. Proc Natl Acad Sci USA. 106:570–575. 2009. View Article : Google Scholar : PubMed/NCBI
|