Introduction
Growing tumor tissues require nutrients, which are
supplied by the increased blood flow caused by tumor angiogenesis.
However, the glucose level is frequently reduced in local tumor
tissues (1,2), suggesting that proliferating cancer
cells are exposed to nutrient deprivation. Numerous previous
studies have shown how cancer cells survive under these conditions
(3–6).
It is generally suggested that cancer growth causes
a host immune response, although it is often insufficient for
immune function. Various mechanisms have been proposed to explain
this immunosuppression, including the inhibition of T-cell function
by immunosuppressive factors (7–10). T
lymphocytes produce various cytokines, which serve important roles
in the immune system. Completely differentiated T-helper (Th)
lymphocytes are divided into at least two subtypes, Th1 and Th2.
Th1 lymphocytes secrete cytokines, including interleukin (IL)-2 and
interferon (IFN)-γ, which activate cytotoxic T lymphocytes and
macrophages, and also tumor necrosis factor β, which is associated
with tumor injury. Th2 cells secrete cytokines, including IL-4,
IL-5 and IL-6, which induce B cell differentiation, which is
associated with antibody production (11–15).
Th1/Th2 imbalance has been reported to be associated with various
diseases. For example, Th1 predominance is reported in rheumatoid
arthritis (16), whereas Th2
predominance is reported in certain neoplastic diseases, including
basal cell carcinoma (17,18), multiple myeloma (19), and colon cancer (20,21).
However, the mechanisms underlying these imbalances remain to be
elucidated.
While cancer cells proliferate, immune cells are
also exposed to the identical condition of nutrient deprivation. It
remains to be investigated how nutrient deprivation affects immune
cells. One possible mechanism of cancer-mediated immunosuppression
is that starved microenvironments have a detrimental effect on the
proliferation and cytokine secretion by immune cells. To assess
this hypothesis, the present study examined how peripheral blood
lymphocytes (PBLs), obtained from healthy donors, proliferate under
nutrient deprivation and whether this environment affects the
secretion of intracellular cytokines by PBLs.
Materials and methods
Cells and culture. PBLs were prepared from the
venous blood of five healthy donors, which included two women and
three men with a mean age of 39 years (range, 32–53 years). PBLs
were isolated by density centrifugation on Ficoll-Paque PLUS
(Amersham Biosciences, Buckinghamshire, UK) following washing three
times (250 × g; 180 × g; 120 × g for 10 min) with Dulbecco's
modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA).
The cells were suspended in 0.1% bovine serum albumin (BSA;
Sigma-Aldrich) and antibiotics (Sigma-Aldrich), plus DMEM
containing various glucose concentrations. The cells were cultured
in 96- or 6-well plates coated with 5 µg/ml mouse anti-human
cluster of differentiation (CD)3 monoclonal antibody (Bay
Bioscience, Kobe, Japan) at 4°C overnight to select for T cells.
The cells were subsequently washed with DMEM three times prior to
culturing at 37°C in a humidified atmosphere containing 5%
CO2.
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS)-based cell growth assay. The PBLs were seeded onto
anti-CD3-coated 96-well, flat-bottomed microtiter plates at a
density of 2×105 cells/well and cultured in DMEM,
supplemented with 0.1% BSA and antibiotics, containing glucose at
various concentrations (0, 25, 50, 100, 225 and 450 mg/dl). The
cells were cultured at 37°C under a humidified atmosphere
containing 5% CO2 for 3 days. The PBLs were subsequently
incubated for 2 h with MTS solution, containing the
electron-coupling reagent, phenazine methosulfate (Promega KK,
Tokyo, Japan), at a final concentration of 10%. Following a 2 h
incubation period, cell vitality was assessed by measuring the
absorbance (490 nm) of each well using a microplate reader
(ImmunoMini NJ-2300; Japan Inter Med Co., Ltd., Tokyo, Japan).
Monoclonal antibodies (mAb). Fluorescein
isothiocyanate (FITC)-conjugated mouse anti-human CD4 mAb (cat. no.
555346), phycoerythrin (PE)-conjugated mouse anti-human IL-4 mAb
(cat. no. 559333) and phycoerythrin (PE)-conjugated IFN-γ mAb (cat.
no. 554701; all mouse IgG1), and FITC-mouse IgG1 (cat. no. 349041)
and PE-mouse IgG1 (cat. no. 349043) were purchased from Becton
Dickinson (San Jose, CA, USA).
Staining and flow cytometry. PBLs were seeded into
anti-CD3-coated 6-well flat-bottomed microtiter plates in DMEM,
containing either 450 mg/dl or no glucose, supplemented with 0.1%
BSA and antibiotics at a density of 1×107 cells/well.
The cells were cultured at 37°C under a humidified atmosphere
containing 5% CO2. Following culturing for 3 days, the
cells were suspended in DMEM containing either 450 mg/dl or no
glucose, supplemented with 0.1% BSA, antibiotics and a 2 µM final
concentration of GolgiStop, an intracellular protein transport
inhibitor containing monensin (Becton Dickinson) for 6 h. The cells
were subsequently incubated with 50 ng/ml phorbol 12-myristate
13-acetate (PMA; Sigma-Aldrich) and 250 ng/ml ionomycin
(Sigma-Aldrich) under an atmosphere containing 5% CO2 at
37°C. Following incubation for 6 h, the cells were harvested and
washed once with phosphate-buffered saline. The staining of cell
surface antigens, including CD4, was performed prior to fixation
and permeabilization for staining of intracellular cytokines. The
cells were suspended in a fixation buffer (BioLegend, San Diego,
CA, USA) at room temperature for 20 min. Following centrifugation
at 350 × g for 5 min at 37°C, the fixed cells were resuspended in a
permeabilization wash buffer (BioLegend) and centrifuged twice at
350 × g for 5 min. Following permeabilization, the cells were
stained with mAbs-targeting intracellular cytokines, including IL-4
and IFN-γ. The cells were analyzed by flow cytometry (FACSCalibur;
BD Biosciences, Franklin Lakes, NJ, USA). Lymphocytes were gated by
forward scatter and side scatter, and then CD4(+) cells were gated
by FITC intensity. The cytokine expression ratio in the CD4(+)
cells was determined using the PE intensity.
Statistical analysis. All statistcal analyses were
performed using SPSS version 16.0 (IBM SPSS, Chicago, IL, USA). The
data were analyzed for statistical significance using the Tukey's
test and Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
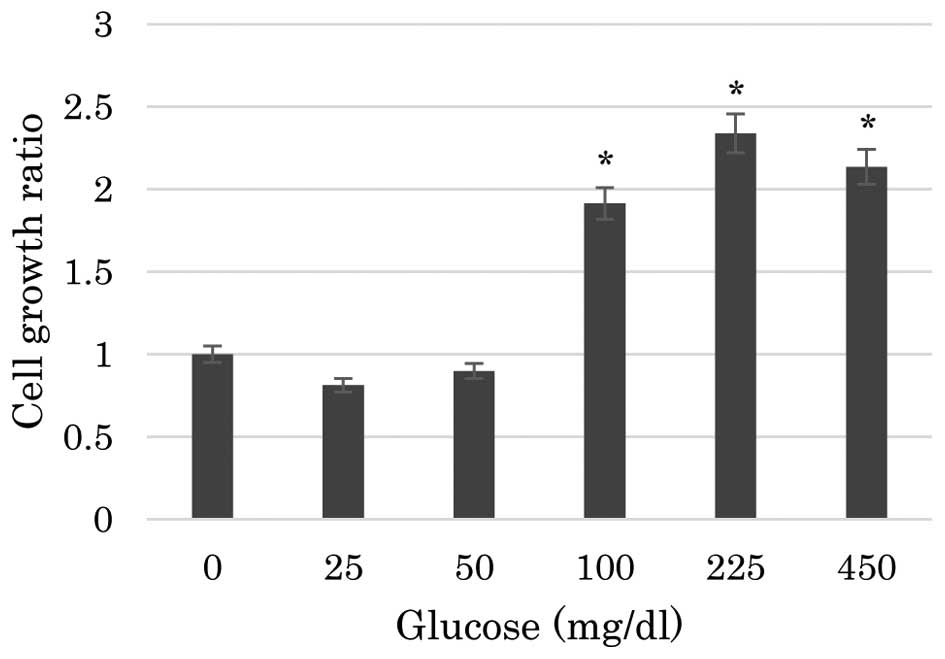
The present study first assessed the effects of
nutrient deprivation on the proliferation of PBLs using an MTS
assay. As shown in Fig. 1, the
proliferation of PBLs was significantly reduced in media containing
no glucose or a low glucose level compared with that in media
containing ≥100 gm/dl glucose. The proliferation of PBLs in media
containing 25 or 50 mg/dl glucose was significantly reduced to the
identical level as that in media containing 0 mg/dl glucose
(P<0.001). The differences in the proliferation of PBLs among
the media containing 0, 25 or 50 mg/dl glucose were not
statistically significant. Similarly, the proliferation of PBLs was
not significantly different in media containing 100, 225 or 450
mg/dl glucose.
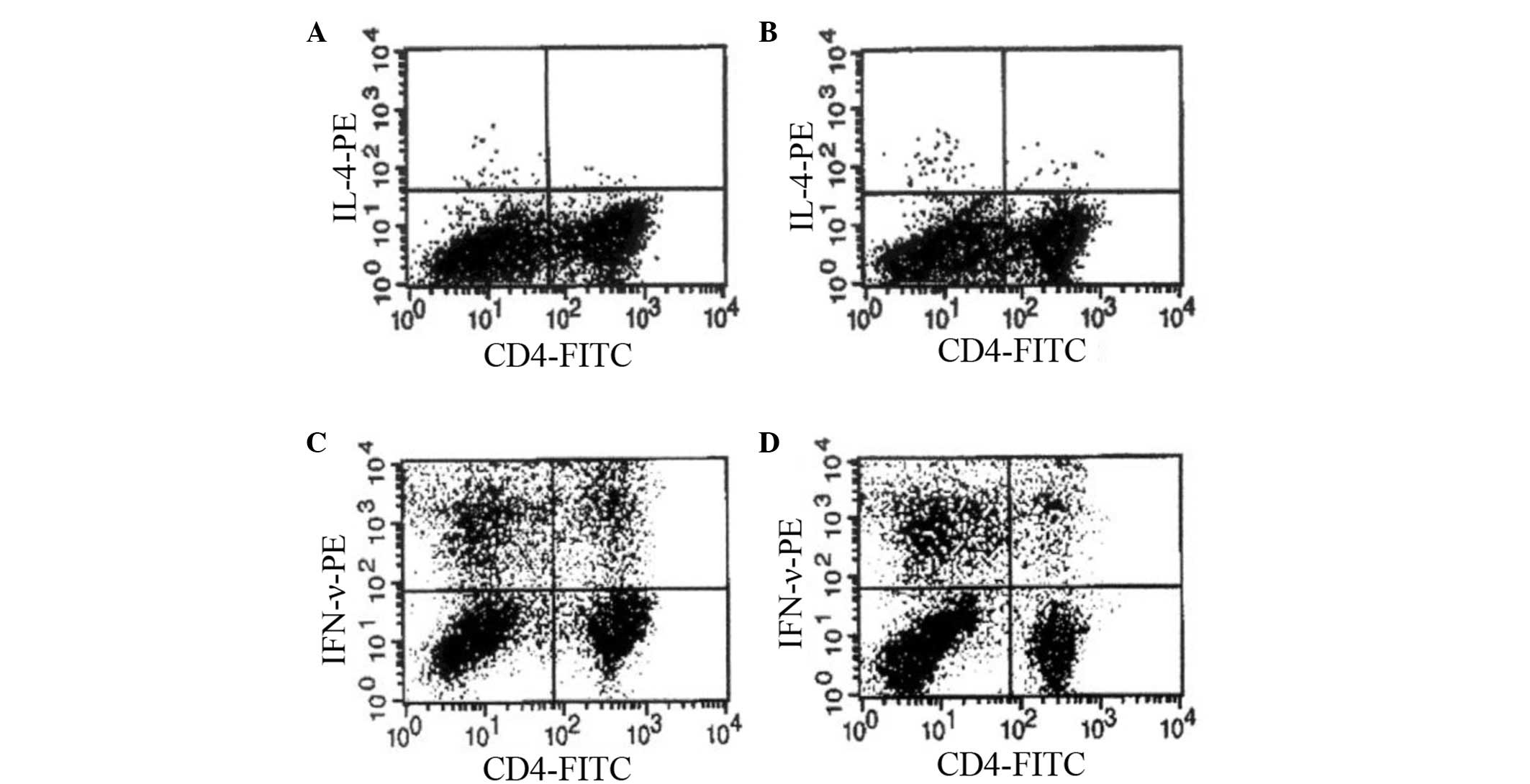
Next, the present study compared cytokine secretion
by PBLs cultured in a high glucose-containing medium and a
glucose-deprived medium. The immunostaining patterns of
intracellular cytokines induced by the activation with PMA and
ionomycin were examined in CD4(+) cell populations. The frequency
of cytokine-positive cells reached a maximum following 6 h of
stimulation and gradually decreased thereafter (data not shown).
Therefore, the cytokine expression following 6 h of stimulation was
examined in subsequent experiments. Fig.
2 demonstrates the representative dot plot analyses of
intracellular cytokines following culturing with or without high
levels of glucose. The percentages of cytokine-positive cells in
CD4(+) T-cell populations are listed in Table I. Cells cultured in no-glucose medium
contained a significantly higher percentage of IL-4-positive CD4(+)
T cells compared with those cultured in high-glucose medium
(0.30±0.05 vs. 0.12±0.10%; P=0.049). However, the percentage of
IFN-γ-positive CD4(+) T cells significantly decreased in cells
cultured in no glucose medium compared with those cultured in
high-glucose medium (6.39±0.56 vs. 15.37±3.14%; P=0.008).
 | Table I.Percentage of cytokine-positive cells
among CD4(+) peripheral blood lymphocytes cultured in high or no
glucose medium following stimulation with phorbol 12-myristate
13-acetate and ionomycin. |
Table I.
Percentage of cytokine-positive cells
among CD4(+) peripheral blood lymphocytes cultured in high or no
glucose medium following stimulation with phorbol 12-myristate
13-acetate and ionomycin.
|
| Percentage of
positive cells |
|---|
|
|
|
|---|
| Glucose | Interleukin-4 | Interferon-γ |
|---|
| High | 0.12±0.10 | 15.37±3.14 |
| No |
0.30±0.05a |
6.39±0.56a |
Discussion
The present study demonstrated that low glucose
levels in the culture medium had a detrimental effect on the
proliferation of PBLs. The majority of previous studies have
reported that nutrient deprivation affects the growth of cancer
cells (4,5); however, immune cells exposed to the
identical conditions remain to be examined in detail. In the
present data, the proliferation of PBLs in media containing glucose
concentrations less than the standard blood sugar (BS) level was
significantly restrained compared with that in the media containing
glucose concentrations of the standard BS level or higher. The
proliferation ratio of PBLs was not directly proportional to the
glucose level of the medium. It appeared that standard BS level was
a boundary; media containing glucose concentrations that were
higher or lower than the standard BS level resulted in different
levels of cell growth. In vivo, cancer cells tend to
colonize and establish angiogenesis to supply energy. However, it
is hypothesized that insufficient vascular structure produces local
nutrient-starved microenvironments. Therefore, it is possible that
local glucose deficiencies interfere with immune cell function,
making it ineffective against cancer.
Furthermore, the present study demonstrated that
nutrient starvation affected PBL cytokine secretion. Several
previous studies have reported that certain diseases exhibit a
Th1/Th2 imbalance, and Th2 predominance has been observed in
certain neoplastic diseases (20,21). A
previous study revealed that IL-2 and IFN-γ were downregulated in
PBLs of patients with gastrointestinal cancer (22). Another previous study demonstrated
that the frequency of IL-4-producing cells only slightly increased
in CD4(+) peripheral blood mononuclear cells of various patients
with cancer, whereas the frequency of IFN-γ-producing cells
decreased (23). The present
experiments indicated that glucose deficiency fostered the
secretion of IL-4 and interfered with the secretion of IFN-γ in
CD4(+) PBLs of healthy donors. Th2 cells secreted IL-4; therefore,
the increased frequency of IL-4-producing cells suggests an
increase in the number of Th2 cells, at least in part. By contrast,
Th1 cells secreted IFN-γ; therefore, the decreased frequency of
IFN-γ-producing cells may be a result of a decrease in the number
of Th1 cells. The present results are consistent with previous
reports revealing Th2 predominance in malignant diseases. Although
the reason for Th2 predominance in neoplastic diseases remains to
be elucidated, it is possible that glucose-deficient
microenvironments in local cancer tissues are associated with Th2
predominance in malignant diseases. Th2 predominance or Th1
dysfunction means that the cytotoxic immune system does not
effectively function, and this is hypothesized to be advantageous
for cancer growth.
The present study attempted to determine the
association between immune cells and their surroundings,
particularly glucose suppression. However, the environment
encircling immune cells comprises various conditions, including
nutrient depression and hypoxia. Proliferating cancer cells require
oxygen and nutrients; however, tumor vessels cannot supply them
with sufficient oxygen (1,2). In addition, nutrient starvation is
associated with various factors, including glucose, amino acids and
serum. These factors are hypothesized to affect the growth and
functions of immune cells. Therefore, limitations exist to the
explanation of immunosuppression by glucose depression alone.
Although the present study regarded proliferation and cytokine
secretion of immune cells as indexes of immune system function,
immune cells have other diverse functions and further research is
required. Furthermore, a fundamental limitation to the present
study is the possibility that PBLs from healthy donors are
different from PBLs from patients with cancer.
In conclusion, glucose deficiency has an effect on
the proliferation of PBLs and their secretion of cytokines. The
proliferation of PBLs cultured in medium containing <100 mg/dl
glucose was significantly reduced compared with the proliferation
of PBLs cultured in a medium containing a standard blood sugar
level or greater. Furthermore, a glucose-deprived environment
increases the frequency of IL-4-producing cells among the CD4(+)
PBLs of healthy donors and decreases the frequency of
IFN-γ-producing cells. These results indicated that the local
immunosuppressive conditions and Th2 predominance in malignant
diseases are partly due to the microenvironment surrounding cancer,
including glucose deprivation.
References
|
1
|
Jain RK: Molecular regulation of vessel
maturation. Nat Med. 9:685–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vaupel P, Thews O and Hoeckel M: Treatment
resistance of solid tumors: Role of hypoxia and anemia. Med Oncol.
18:243–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park HR, Tomida A, Sato S, Tsukumo Y, Yun
J, Yamori T, Hayakawa Y, Tsuruo T and Shin-ya K: Effect on tumor
cells of blocking survival response to glucose deprivation. J Natl
Cancer Inst. 96:1300–1310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He L, Li X, Luo HS, Rong H and Cai J:
Possible mechanism for the regulation of glucose on proliferation,
inhibition and apoptosis of colon cancer cells induced by sodium
butyrate. World J Gastroenterol. 13:4015–4018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato K, Tsuchihara K, Fujii S, Sugiyama M,
Goya T, Atomi Y, Ueno T, Ochiai A and Esumi H: Autophagy is
activated in colorectal cancer cells and contributes to the
tolerance to nutrient deprivation. Cancr Res. 67:9677–9684. 2007.
View Article : Google Scholar
|
|
6
|
Ferraro E and Cecconi F: Autophagic and
apoptotic response to stress signals in mammalian cells. Arch
Biochem Biophys. 462:210–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naor D: Suppressor cells: Permitters and
promoters of malignancy? Adv Cancer Res. 29:45–125. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fearon ER, Pardoll DM, Itaya D, Golumbek
P, Levitsky HI, Simons JW, Karasuyama H, Vogelstein B and Frost P:
Interleukin-2 production by tumor cells bypasses T helper function
in the generation of an antitumor response. Cell. 60:397–403. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tada T, Ohzeki S, Utsumi K, Takiuchi H,
Muramatsu M, Li XF, Shimizu J, Fujiwara H and Hamaoka T:
Transforming growth factor-beta-induced inhibition of T cell
function. Susceptibility difference in T cells of various
phenotypes and functions and its relevance to immunosuppression in
the tumor-bearing state. J Immunol. 146:1077–1082. 1991.PubMed/NCBI
|
|
10
|
Gallimore A and Sakaguchi S: Regelation of
tumour immunity by CD25+T cells. Immunology. 107:5–9. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mosmann TR, Cherwinski H, Bond MW, Giedlin
MA and Coffman RL: Two types of murine helper T cell clone. I.
Definition according to profiles of lymphokine activities and
secreted proteins. J Immunol. 136:2348–2357. 1986.PubMed/NCBI
|
|
12
|
Bottomly K: A functional dichotomy in CD4+
T lymphocytes. Immunol Today. 9:268–273. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mossman TR and Coffman RL: TH1 and TH2
cells: Different patterns of lymphokine secretion lead to different
functional properties. Annu Rev Immunol. 7:145–173. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kupfer A and Singer SJ: Cell biology of
cytotoxic and helper T cell functions. Annu Rev Immunol. 7:309–337.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Romagnani S: Lymphokine production by
human T cells in dease state. Annu Rev Immunol. 12:227–257. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berner B, Akça D, Jung T, Muller GA and
Reuss-Borst MA: Analysis of Th1 and Th2 cytokines expressing CD4+
and CD8+ T cells in rheumatoid arthritis by flow cytometry. J
Rheumatol. 27:1128–1135. 2000.PubMed/NCBI
|
|
17
|
Kim J, Modlin RL, Moy RL, Dubinett SM,
McHugh T, Nickoloff BJ and Uyemura K: IL-10 production in cutaneous
basal and squamous cell carcinoma. A mechanism for evading the
local T cell immune response. J Immunol. 155:2240–2247.
1995.PubMed/NCBI
|
|
18
|
Elsässer-Beile U, von Kleist S, Stähle W,
et al: Cytikine levels in whole blood cell cultures as parameters
of the cellular immunologic activity in patients with malignant
melanoma and basal cell carcinoma. Cancer. 71:231–236. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hata H, Xiao H, Petrucci MT, Woodliff J,
Chang R and Epstein J: Interleukin-6 gene expression in multiple
myeloma: A characteristic of immature tumor cells. Blood.
81:3357–3364. 1993.PubMed/NCBI
|
|
20
|
Pellegrini P, Berghella AM, Del Beato T,
Cicia S, Adorno D and Casciani CU: Disregulation in TH1 and TH2
subsets of CD4+ T cells in peripheral blood of colorectal cancer
patients and involvement in cancer establishment and progression.
Cancer Immunol Immunother. 42:1–8. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berghella AM, Pellegrini P, Del Beato T,
Marini M, Tomei E, Adorno D and Casciani CU: The significance of an
increase in soluble interleukin-2 receptor level in colorectal
cancer and its biological regulating role in the physiological
switching of the immune response cytokine network from TH1 to TH2
and back. Cancer Immunol Immunother. 45:241–249. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakayama H, Kitayama J, Muto T and Nagawa
H: Characterization of intracellular cytokine profile of CD4(+) T
cells in peripheral blood and tumor-draining lymph nodes of
patients with gastrointestinal cancer. Jpn J Clin Oncol.
30:301–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato M, Goto S, Kaneko R, Ito M, Sato S
and Takeuchi S: Impaired production of Th1 cytokines and increased
frequency of Th2 subsets in PBMC from advanced cancer patients.
Anticancer Res. 18:3951–3955. 1998.PubMed/NCBI
|