Introduction
Although the restoration of blood flow to ischemic
organs is essential to prevent irreversible tissue damage,
reperfusion is able to trigger local and systemic inflammation,
which leads to more severe tissue injury than ischemia alone
(1). Such ischemia-reperfusion (I/R)
injury may occur in a variety of clinical contexts, including
thrombolytic therapy, coronary angioplasty, organ transplantation,
aortic cross-clamping and cardiopulmonary bypass (1). Several approaches have been applied in
the clinic to treat I/R injury (2),
with perhaps the best-studied example being ischemic
preconditioning (3). This involves
inducing discontinuous ischemic episodes prior to the sustained
ischemia.
Hypoxia-inducible factor-1 (HIF-1), comprising
HIF-1α and HIF-1β subunits (4), is a
central regulator of oxygen homeostasis. HIF-1α is universally
expressed in vertebrates, insects, worms and other species
(5). Under conditions of normoxia
(normal oxygen), the ubiquitin-proteasome pathway maintains levels
of HIF-1α at a minimum (6); in
hypoxia, however, HIF-1α is protected from proteolysis (1), leading to a rapid increase in protein
levels. HIF-1β, by contrast, is expressed constitutively at similar
levels under normal and hypoxic conditions (7).
HIF-1α activates the transcription of genes encoding
proteins that mediate adaptive responses to hypoxia/ischemia,
including erythropoietin, glucose transporter 1, vascular
endothelial growth factor (VEGF), as well as genes regulating cell
survival (1). HIF-1α may also help
drive angiogenesis following tissue injury (8). The length of time during which HIF-1α
continues to influence the expression of downstream effectors
following I/R has yet to be fully elucidated, which is important
for understanding how HIF-1α mitigates I/R injury. In the present
study, the time-scale of HIF-1α action was investigated using a
culture model of myocardial cells derived from Sprague-Dawley
rats.
Materials and methods
Animals
The study protocol was approved by the Animal and
Ethics Review Committee at the Life Science Institute of Sichuan
University in Chengdu, China. A total of 30 male Sprague-Dawley
rats were purchased from the Animal Experiment Center of Sichuan
University (animal license SCXK2006-010). The rats were kept under
light conditions, and they were fed on normal rat fodder provided
by the Animal Experiment Center of Sichuan University.
Construction of a eukaryotic
expression plasmid encoding rat myocardial HIF-1α
Pspt18, a plasmid expressing full-length rat HIF-1α,
empty expression vector pcDNA3.1 (Life Technologies; Thermo Fisher
Scientific, Waltham, MA, USA) and Escherichia coli JM109 were
kindly given by Professor Yi Qu of West China Second University
Hospital (Chengdu, China). The HIF-1α-coding region was subcloned
into pcDNA3.1 using BamHI (Takara Biotechnology Co., Ltd.,
Dalian, China) and DNA ligase Sol l (Takara Biotechnology Co.,
Ltd.), and the resulting expression plasmid was called pHIFα.
Colonies apparently positive for inserts were screened by
BamHI to confirm the presence of the insert.
Myocardial culture model of I/R
injury
Myocardial cells were isolated from Sprague-Dawley
rats, as previously described (2).
Briefly, the pericardium and atrium were removed from 10 rat hearts
in phosphate-buffered saline (PBS) buffer, and ventricular cells
were separated using trypsin protease (Thermo Fisher Scientific).
The cell suspension was passed through a 200 µm screen into culture
flasks. Cultures were maintained for 4 days under normal conditions
(37°C, 5% CO2), prior to exposure for 30 min to hypoxic
conditions (37°C, 95% N2, 5% CO2) to simulate
ischemia. After 24 h, the culture medium was replaced with
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS) to simulate reperfusion (Sigma-Aldrich, St.
Louis, MO, USA). In parallel, mock-I/R control cultures were
maintained under normal conditions (37°C, 5% CO2) for
the same overall culture period. Cultures were harvested (by
trypsin treatment and centrifugation at 1,000 rpm for 5 min) at 6
h, or on day 5 following I/R.
Microscopy
Images were captured using a Nikon Eclipse Ti
microscope (Nikon, Tokyo, Japan) at 100× magnification.
Transient transfections prior to
I/R
Ventricular myocytes were prepared as described
above, and cultured on 30-mm dishes until 80% confluence was
reached. Subsequently, the medium was changed to DMEM without serum
or antibiotics, and the cultures were transfected for 24 h with a
mixture of 8 µg pHIFα and 40 µl Lipofectamine™ (Invitrogen; Thermo
Fisher Scientific). The cultures were subsequently grown in
Dulbecco's modified Eagle's medium containing 10% FBS, and 100
units/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich). In
parallel, control cultures were mock-transfected with
Lipofectamine™ alone. At 1 day following transfection, these
transfected cultures were subjected to I/R or mock-I/R, as
described above.
Measurement of myocardial cell
viability using the methyl thiazolyl tetrazolium (MTT) assay
At 6 h, or 5 days following I/R, cultures were
harvested, resuspended in fresh medium and pelleted by
centrifugation as previously described. The pellets were
resuspended and inoculated in 96-well plates at a density of
5×103 cells per well. Plates were incubated for 24 h,
after which 20 µl MTT reagent (5 mg/ml, Sigma-Aldrich) was added to
each well. The plates were incubated for a further 4 h, and
subsequently the supernatant was removed. Dimethylsulfoxide (150
µl; Sigma-Aldrich) was added to each well. The absorbance was
measured at 490 nm using a Bio-Rad iMark™ microplate reader
(Bio-Rad Laboratories, Inc.).
Western blotting to measure the
protein expression levels of HIF-1α and VEGF
The cells were directly washed three times with PBS,
and lysed in sodium dodecyl sulfate (SDS) sample buffer [50 mM
Tris-HCl (pH 6.8), 1% SDS, 10% glycerol, 5% β-mercaptoethanol,
0.01% Bromophenol blue; all reagents purchased from Sigma-Aldrich].
Cell lysates were boiled for 5 min.
The supernatants were analyzed by SDS-polyacrylamide
gel electrophoresis (10% gels, run at 85 V for 45 min, and then at
135 V for 1 h), and subsequently transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). The filters were blocked
with 5% milk, followed by incubation with a monoclonal antibody
against HIF-1α (1:250), VEGF (1:250) or β-actin (1:250; all
antibodies purchased from ZSGB-BIO, Beijing, China). Binding of the
antibodies was detected using the ChemiDoc™ XRS system (Bio-Rad
Laboratories, Inc.).
Flow cytometric analysis to measure
apoptosis
Cells were harvested by treatment with trypsin
protease (Thermo Fisher Scientific) with subsequent washing with
PBS, and then were fixed with ethanol (70%) and maintained at 4°C
for 30 min. The cells were again pelleted as previously described,
the fixation fluid was removed, and the cells were resuspended in
50 µl propidium iodide (0.4 mg/ml). Labeled cells were subsequently
analyzed by flow-activated cell sorting using a Becton-Dickson
Model 420 Fluorescence-Activated Cell Sorter (BD Biosciences,
Bedford, MA, USA).
Statistical analyses
The results for cells harvested at different times
following I/R with or without prior transfection were analysed by
making a comparison of the mean ± standard deviation. Statistical
analyses were performed using SPSS version 19.0 software (IBM SPSS,
Armonk, NY, USA). P<0.05 was taken to indicate a statistically
significant value.
Results
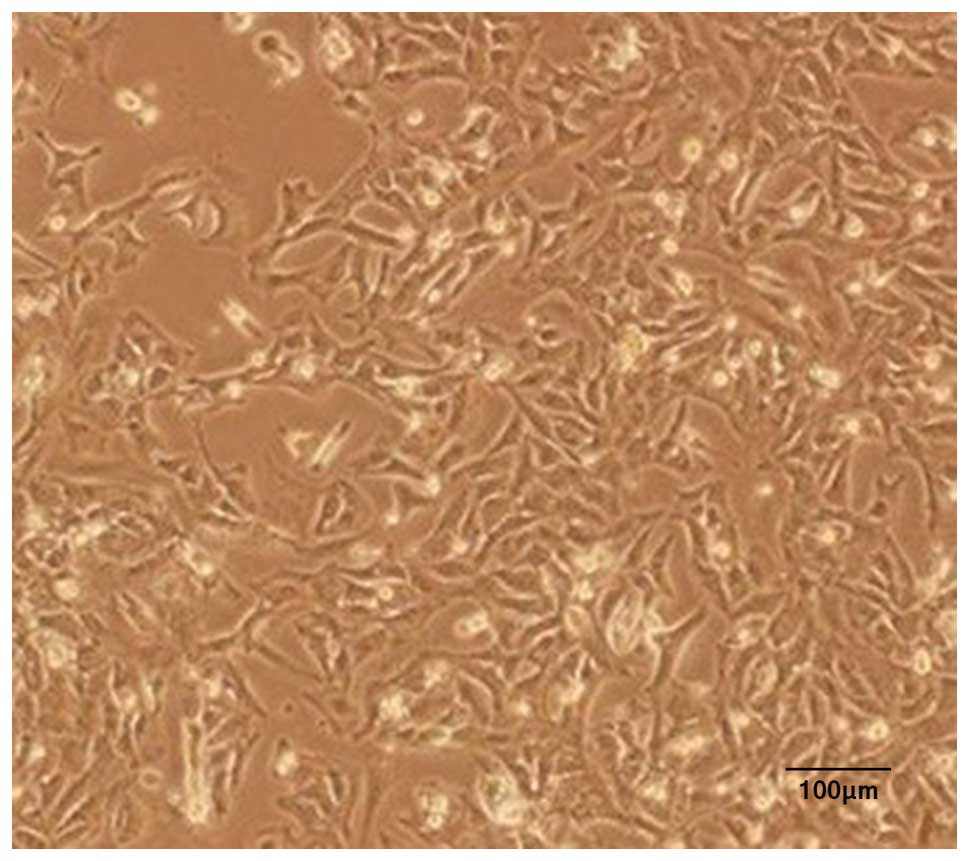
Microscopy of the cultures
Inverted microscopy of cultures of the rat
myocardial cells revealed the expected morphology of prismatic, or
irregular, triangles with ovaloid nuclei centered in the cells
(Fig. 1). Analysis of the myocardial
cells in DMEM culture dishes showed a pulsation frequency of 100±10
beats/min.
Influence of the expression levels of
HIF-1a on myocardial cell viability following I/R
At 6 h following I/R, cell viability was markedly
lower in cells subjected to I/R compared with control cells not
subjected to I/R, irrespective of whether the cells were
untransfected or transfected with empty vector (Fig. 2). Cell viability was markedly higher
in cultures transiently transfected with pHIF-1α. At 5 d following
I/R, the viability of these cells was much lower than at 6 h, and
was only slightly higher than the viability of untransfected cells
and cells transfected with empty vector.
Changes in the expression levels of
VEGF and HIF-1a following I/R
HIF-1α has been shown to upregulate the expression
of VEGF in response to hypoxia. In the culture system utilized in
the present study, at 6 h following I/R, the levels of HIF-1α and
VEGF were higher in cultures treated with I/R compared with
mock-treated control cells (Fig. 3).
Levels were similar at 6 h for untransfected cells and cells
transiently transfected with pHIF-1α prior to I/R. At 5 days
following I/R, the levels of HIF-1α remained higher in I/R-treated
cells compared with the mock-treated cells. Conversely, the levels
of VEGF were markedly lower in I/R-treated cells compared with
mock-treated ones.
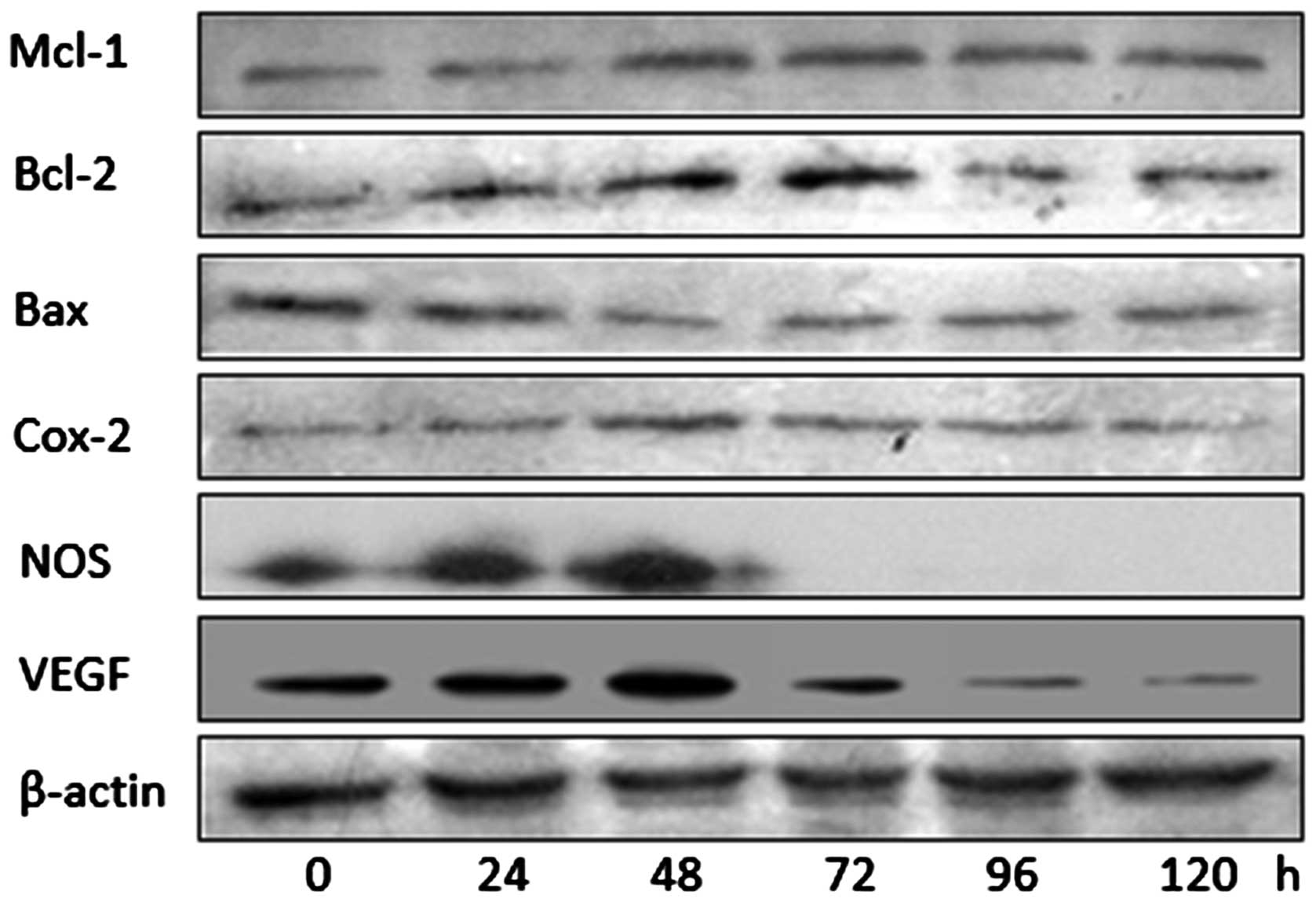
Western blotting of effector proteins
downstream of HIF-1α
The results in Fig. 3
suggested that the hypoxia-induced upregulation of HIF-1α led to
short-term (6 h), but not long-term (5 days), upregulation of VEGF
expression. Consequently, it was hypothesized whether the same may
also be true for other downstream effector molecules of HIF-1α.
Therefore, western blotting was used to assess the levels of B-cell
lymphoma-2 (Bcl-2), myeloid cell leukemia 1 (Mcl-1),
Bcl-2-associated × protein (Bax), cyclo-oxygenase 2 (Cox-2), nitric
oxide synthase (NOS), as well as VEGF at numerous times between 0
and 120 h following I/R in untransfected cells (Fig. 4). These results suggested that,
although levels of Bax decreased for much of the time course and
potentially increased later, the levels of Mcl-1, Bcl-2, Cox-2, NOS
and VEGF peaked at 48–72 h, after which they decreased.
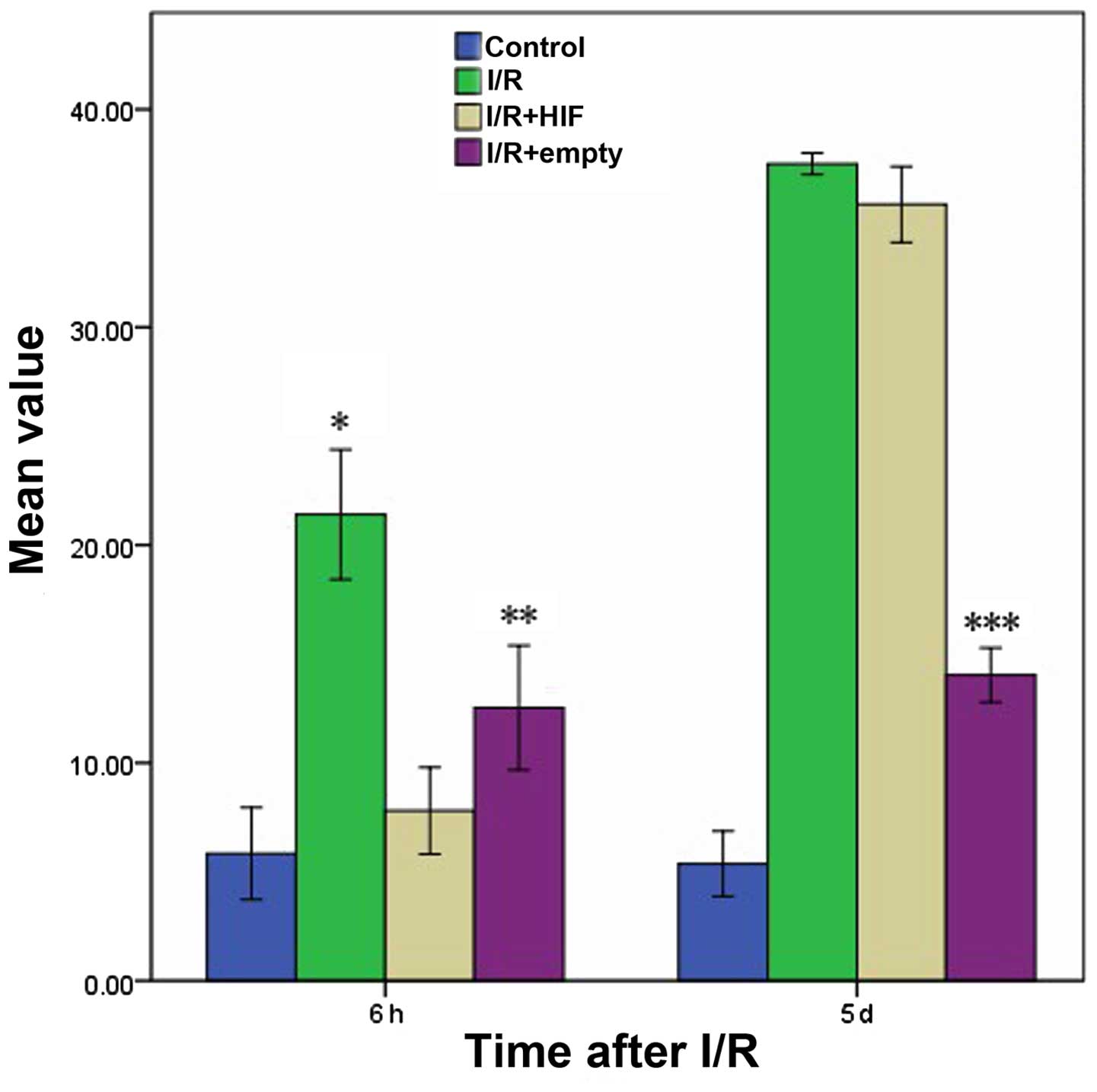
Short-term protection against
apoptosis by HIF-1α
The percentage of apoptotic myocardial cells was
compared following I/R and mock treatment in cells that were
untransfected or transfected prior to I/R with either empty
expression vector or expression plasmid encoding full-length HIF-1α
(Fig. 5). Following treatment with
I/R, at 6 h the I/R+HIF group exhibited a similar level of
apoptosis with the Control group, which was maintained at a low
level compared with the I/R and I/R+empty groups. However, at 5
days following I/R, the proportion of apoptotic myocardial cells in
the HIF overexpression group had reached a high level, comparable
with that of the I/R group.
Discussion
In the present study, it has been shown that, in a
cell culture system that models I/R in animals, the expression of
HIF-1α triggers a short-term upregulation of several downstream
effector molecules associated with tissue repair, and the levels of
these effectors return to the baseline over the longer term. This
occurs even though the levels of HIF-1α remained relatively high at
5 days following I/R. These findings suggest that the
cardioprotective effects of HIF-1α following hypoxia are
temporary.
In contrast with a previous study in mice, which
suggested that the levels of HIF-1α peak soon after hypoxic injury
and subsequently rapidly fall back to the baseline (8), in the present study HIF-1α was
detectable at 5 days following I/R-stimulated upregulation of
HIF-1α. The difference in these results may reflect different
detection systems. The observation that levels of downstream
effectors of HIF-1α decreased faster, and to a greater extent,
compared with the levels of HIF-1α itself suggest that additional
factors or pathways are involved in regulating these downstream
effectors following I/R. It is possible that HIF-1α initially
upregulates these effectors soon after hypoxic injury, and
subsequently other factors regulate them in the longer term.
The effectors upregulated in response to I/R in the
culture system used in the present study are known to help mitigate
the effects of hypoxic injury. Ischemic preconditioning upregulates
Cox-2 levels in the heart, helping to protect the tissue against
the effects of myocardial infarction (9). In endothelial cells, Cox-2-dependent
production of prostacyclin may exert antithrombotic effects
(10), and in myocardial cells,
adiponectin acts via a Cox2-dependent pathway to mitigate I/R
injury (11). NO, which offers one of
the most important defenses against myocardial I/R injury (2), is produced by NOS primarily in the
cardiovascular system, where it helps to regulate apoptosis. Cox-2
and NO interact to provide cardioprotection, since Cox-2 requires
NO for its activity (12). The
results of the present study suggest that the protective effects
provided by NOS and Cox-2 last for only ~48 h, with their levels
approaching the pre-injury baseline following ~72 h (Fig. 4).
These results suggest that HIF-1α in myocardial
cells temporarily increases the expression of the antiapoptotic
proteins, Bcl-2 and Mcl-1, while at the same time inhibiting
expression of the proapoptotic protein, Bax (Fig. 4). This would be consistent with
previous studies, which reported that overexpression of Bcl-2 in
cardiac ischemia suppresses cell death and reduces metabolic
functions of the mitochondria (10).
HIF-1α appears to exert a similarly short-term effect on VEGF, the
levels of which peak at 48–72 h following I/R, and subsequently
rapidly return to baseline. VEGF stimulates neovascularization and
enhances resistance to ischemia (1,13).
In conclusion, the present study provides evidence
that the ability of HIF-1α and its downstream effectors to
attenuate I/R injury in myocardial cells is temporary. These
findings may have implications for treating such injuries in a
variety of clinical contexts.
Acknowledgements
The present study was supported by the Department of
Science and Technology of Sichuan Province, China [grant no.
2011SZ0116] and the Program of Medicine Administration Bureau of
Sichuan Province [grant no. 2012-F-024], as well as the Scientific
and Technological Project of Sichuan Province [grant no. 6zc1671].
We would like to thank Professor Yi Qu of West China Second
University Hospital (Chengdu, China) for her assistance.
References
|
1
|
Eltzschig HK and Collard CD: Vascular
ischaemia and reperfusion injury. Br Med Bull. 70:71–86. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ockaili R, Natarajan R, Salloum F, Fisher
BJ, Jones D, Fowler AA III and Kukreja RC: HIF-1 activation
attenuates postischemic myocardial injury: Role for heme
oxygenase-1 in modulating microvascular chemokine generation. Am J
Physiol Heart Circ Physiol. 289:H542–H548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Epstein AC, Gleadle JM, McNeill LA,
Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI,
Dhanda A, et al: C. elegans EGL-9 and mammalian homologs
define a family of dioxygenases that regulate HIF by prolyl
hydroxylation. Cell. 107:43–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hofer T, Wenger R and Gassmann M: Oxygen
sensing, HIF-1alpha stabilization and potential therapeutic
strategies. Pflugers Arch. 443:503–507. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stroka DM, Burkhardt T, Desbaillets I,
Wenger RH, Neil DA, Bauer C, Gassmann M and Candinas D: HIF-1 is
expressed in normoxic tissue and displays an organ-specific
regulation under systemic hypoxia. FASEB J. 15:2445–2453.
2001.PubMed/NCBI
|
|
6
|
Lipsky PE, Brooks P, Crofford LJ, DuBois
R, Graham D, Simon LS, van de Putte LB and Abramson SB: Unresolved
issues in the role of cyclooxygenase-2 in normal physiologic
processes and disease. Arch Intern Med. 160:913–920. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bolli R, Shinmura K, Tang XL, Kodani E,
Xuan YT, Guo Y and Dawn B: Discovery of a new function of
cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that
alleviates ischemia/reperfusion injury and mediates the late phase
of preconditioning. Cardiovasc Res. 55:506–519. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams JM, Difazio LT, Rolandelli RH, Luján
JJ, Haskó G, Csóka B, Selmeczy Z and Németh ZH: HIF-1: A key
mediator in hypoxia. Acta Physiol Hung. 96:19–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramírez-Bergeron DL, Runge A, Adelman DM,
Gohil M and Simon MC: HIF-dependent hematopoietic factors regulate
the development of the embryonic vasculature. Dev Cell. 11:81–92.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Date T, Belanger AJ, Mochizuki S, Sullivan
JA, Liu LX, Scaria A, Cheng SH, Gregory RJ and Jiang C:
Adenovirus-mediated expression of p35 prevents
hypoxia/reoxygenation injury by reducing reactive oxygen species
and caspase activity. Cardiovasc Res. 55:309–319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibata R, Sato K, Pimentel DR, Takemura
Y, Kihara S, Ohashi K, Funahashi T, Ouchi N and Walsh K:
Adiponectin protects against myocardial ischemia-reperfusion injury
through AMPK- and COX-2-dependent mechanisms. Nat Med.
11:1096–1103. 2005. View
Article : Google Scholar : PubMed/NCBI
|