Introduction
Cytokeratin 19 fragment 21-1 (CYFRA21-1) is a
circulating soluble fragment of keratin 19 (K19) (1). CYFRA21-1 is a marker of lung cancer
useful for evaluating clinical diagnosis and prognosis (2). However, to the best of our knowledge, no
previous cases of cutaneous squamous cell carcinoma (SCC) with high
levels of CYFRA21-1 have been reported to date.
We herein report a case of K19-positive cutaneous
SCC with elevated CYFRA 21-1 levels.
Case report
A 79-year-old man presented to our hospital (Meiwa
Hospital, Nishinomiya, Japan) with a large subcutaneous tumor on
the left shoulder. The patient's past medical history included
duodenal ulcer, appendicitis, liver cirrhosis and hepatitis C. One
month prior to the first visit, the patient noticed a tumor on the
left shoulder exhibiting rapid growth. On clinical examination at
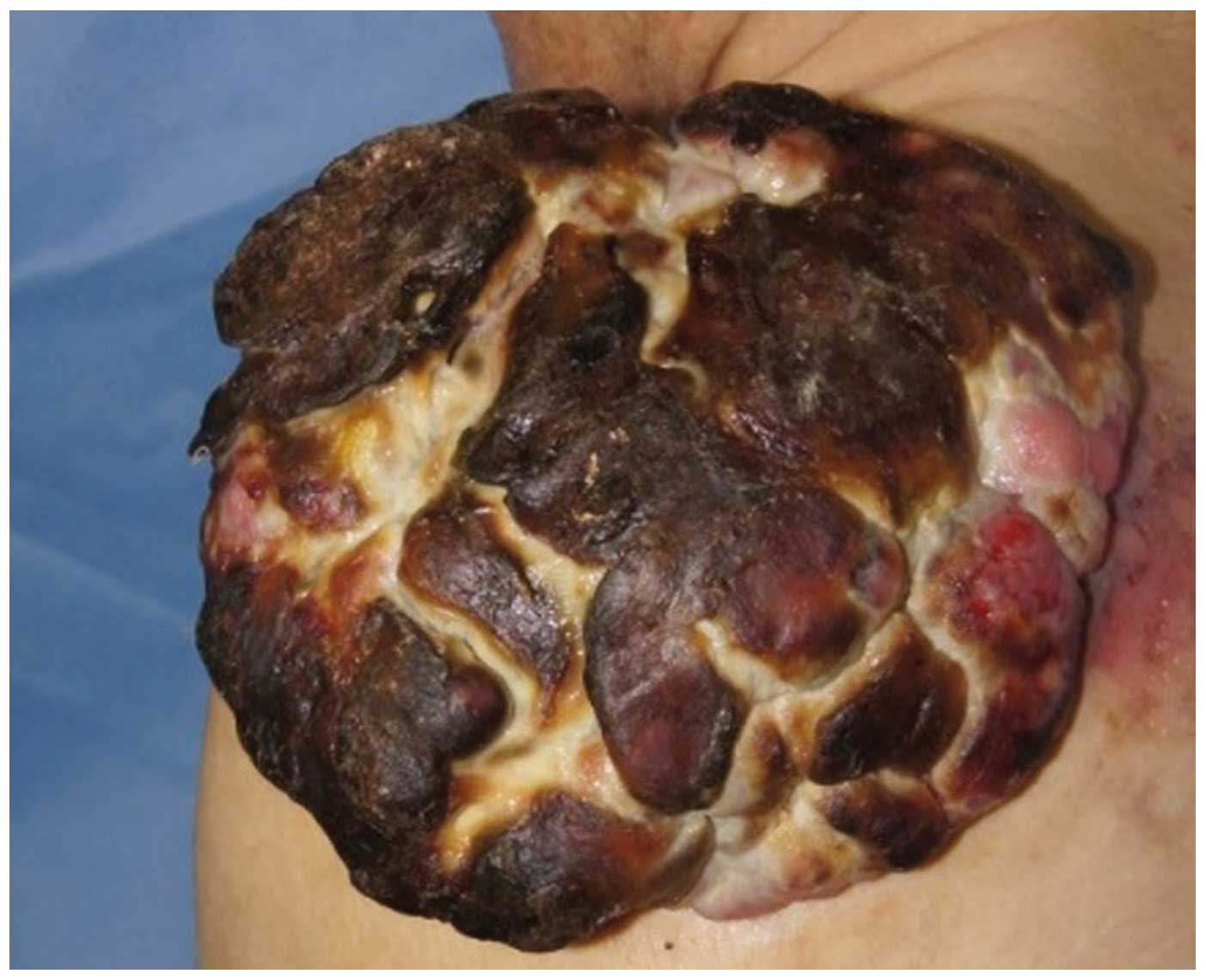
the first visit, the tumor was solid, sized 16×10×5 cm, with a
cauliflower-like appearance and accompanying hemorrhage, necrosis
and ulcerations (Fig. 1).
The laboratory findings included elevated white
blood cell count (13,100/µl) with a left shift, with 86%
neutrophils, and anemia (hemoglobin 5.7 g/dl). Three months prior
to the first visit, the hemoglobin concentration was 10.0 g/dl.
An elevated C-reactive protein level was also found
(6.90 mg/dl). The levels of carcinoembryonic antigen (3.4 ng/ml)
and SCC antigen (1.7 ng/ml) were within normal limits. However, the
level of CYFRA21-1 was significantly elevated (33 ng/ml).
Enhanced magnetic resonance imaging examination
revealed a pedunculated tumor on the deltoid muscle on T2-weighted
images. The normal range of CYFRA21-1 is >3.5 ng/ml. Computed
tomography revealed metastasis to the neck, subclavicular and
mediastinal lymph nodes.
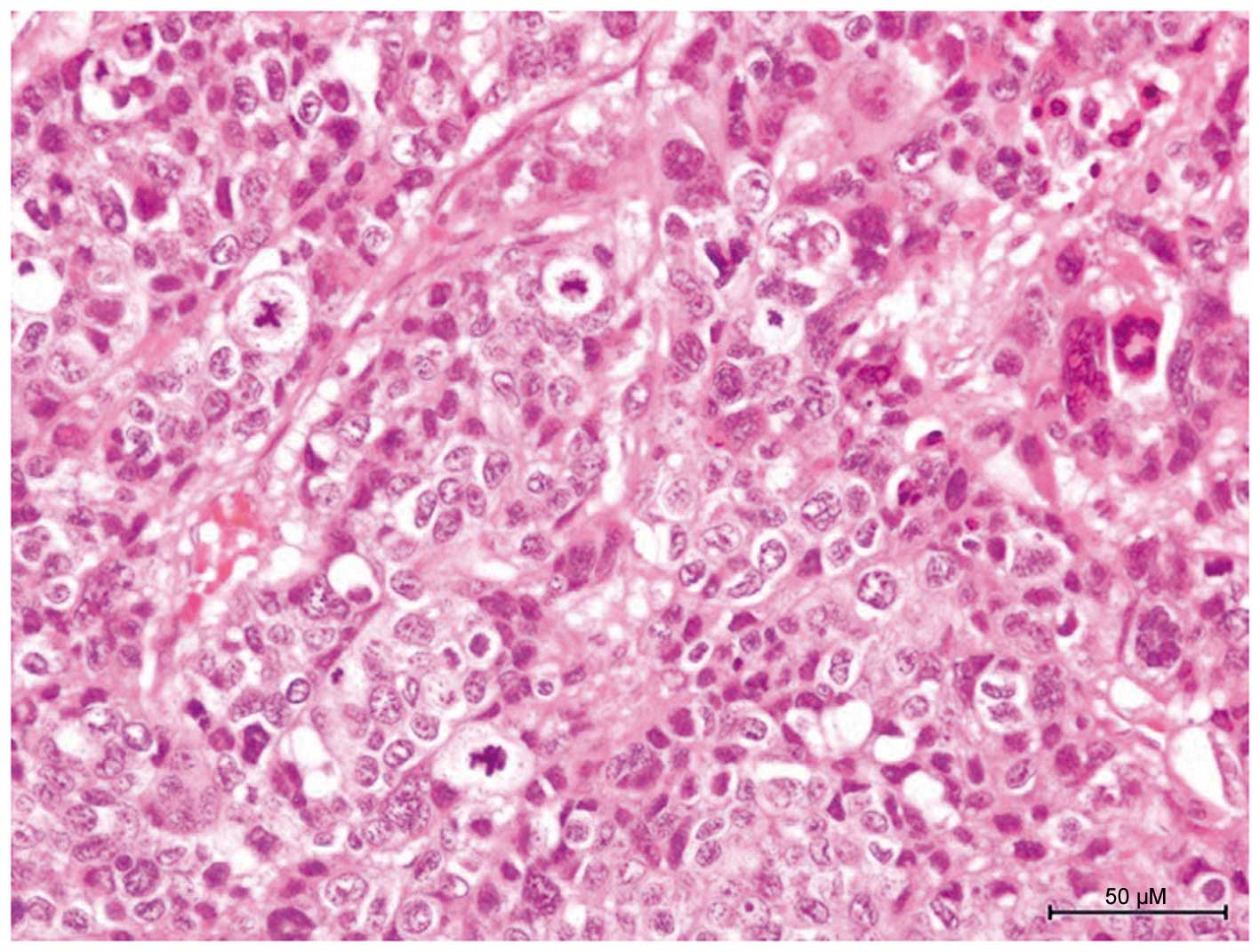
On histopathological examination, tumor nests were
identified in the entire thickness of the dermis. The tumor nests
were continuous with the epidermis and were composed of poorly
differentiated atypical squamous cells exhibiting high-grade
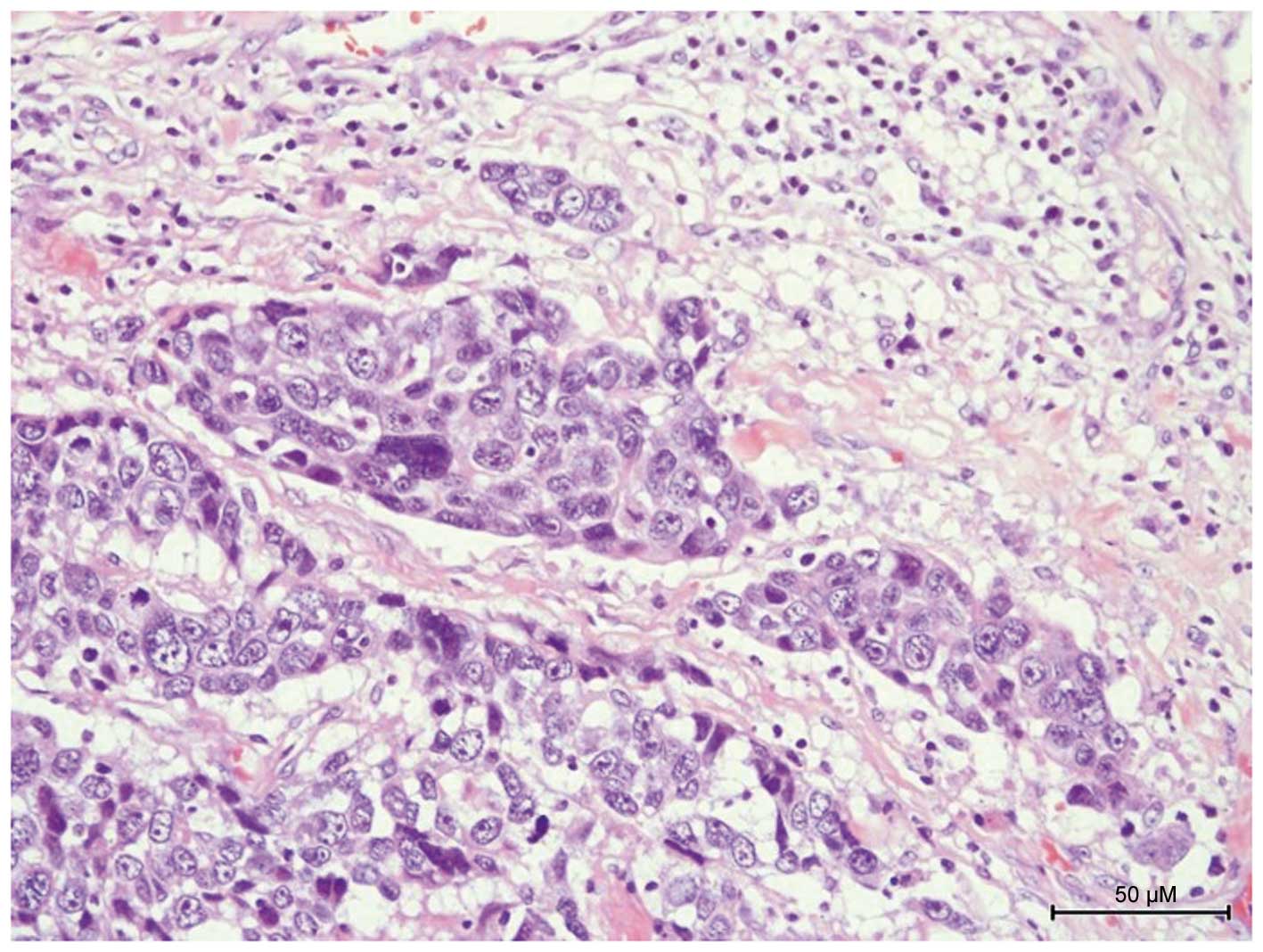
malignancy, with mitotic figures and multinuclear cells (Fig. 2). lymph vessels invaded by poorly
differentiated cutaneous SCC cells were also identified (Fig. 3).
Based on the abovementioned clinical and
histopathological findings, the diagnosis was cT3N2M1 (stage IV)
poorly differentiated primary cutaneous SCC, metastatic to the
regional lymph nodes (left axilla, neck and mediastinum).
The tumor was widely excised, with partial excision
of the deltoid and trapezius muscles. Grafting with a latissimus
dorsi flap was performed with axillary lymph node dissection. three
cycles of postoperative chemotherapy with pepleomycin sulfate (a
total of 85 mg) were administered. Following surgery, the level of
CYFRA21-1 was significantly reduced. Further treatment was not
performed, and the patient's current condition is not known as
there were no follow-up hospital visits.
On immunohistochemical examination for keratin
expression, the labeled streptavidin-biotin (LSAB) method was used
(Dako, Carpinteria, CA, USA). The mouse anti-human keratin
antibodies used in this study were as previously described
(3): 34βB4 (K1; dilution 1:50), LP5K
(K7; dilution 1:10), LP3K (K8; dilution 1:50), HP1 (K10; dilution
1:50), LL002 (K14; dilution 1:200), LHK15 (K15; dilution 1:40),
LL025 (K16; dilution 1:20), E3 (K17; dilution 1:25), 5D3 (K18;
dilution 1:20) and b170 (K19; dilution 1:100), all from Novocastra
Laboratories Ltd., Newcastle upon Tyne, UK. The LSAB method was
applied according to the manufacturer's instructions, as previously
reported (3). K19 was significantly
expressed in tumor cells (Fig. 4). K7
was weakly expressed in tumor nests. K8, K18 and the other keratins
were not expressed in tumor cells.
Patient's written informed consent was obtained for
publication of this case study and the accompanying images.
Discussion
Keratins are the most diverse intermediate
filaments, and may be used as markers of epithelial tumors, stage
of differentiation and origin of epithelial tumors. There are a
total of 54 human functional keratin genes (4).
K19 is the smallest keratin, with a molecular weight
of 40 kd. The CYFRA21-1 circulating fragment is a marker of K19. In
normal skin, K19 is present in the outermost cells of the hair
follicle and in simple ductal epithelia (4); it is also found in lung adenocarcinoma
and SCC (5).
As regards keratin expression in cutaneous SCC,
stratified differentiated keratins (K1 and K10) are commonly
detected in well-differentiated SCC, whereas simple epithelial
keratins (K7, K8, K18 and K19) are detected in poorly
differentiated SCC. These keratins are involved in tumor invasion
and epithelial-mesenchymal interactions (6).
As regards elevated CYFRA21-1 levels in primary
cutaneous carcinoma, two cases of eccrine porocarcinoma were
previously reported (7).
K19 is present in the ductal cells of eccrine sweat
glands. Therefore, the presence of K19 in eccrine sweat glands may
be reflected in elevated CYFRA21-1 in eccrine porocarcinoma. To the
best of our knowledge, no previous cases of cutaneous scc with
elevated CYFRA21-1 have been reported. K19 is not present in the
normal epidermis. Although K19 expression was not detected in blood
vessels, we hypothesized that K19 in tumors is lysed by protease,
resulting in spreading in the tissue, lymph vessels and lymph
nodes. Subsequently, K19 enters blood vessels and is identified as
elevated CYFRA21-1, a soluble cytokeratin fragment. In our case,
CYFRA21-1 level was decreased following surgery. This result may
reflect the clinical prognosis of the tumor. However, further
similar cases should be accumulated in the future.
In summary, we reported a case of K19-positive
cutaneous SCC with elevated serum CYFRA21-1 level. CYFRA21-1 level
may reflect the clinical course of cutaneous SCC and it may be a
marker of primary poorly differentiated cutaneous scc, useful in
the diagnosis and as an indicator of the clinical course.
References
|
1
|
Moll R, Divo M and Langbein L: The human
keratins: Biology and pathology. Histochem Cell Biol. 129:705–733.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ebert W, Bodenmüller H and Hölzel W: CYFRA
21-1-clinical applications and analytical requirements. Scand J
Clin Lab Invest Suppl. 221:72–80. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurokawa I, Nishijima S, Kusumoto K,
Senzaki H, Shikata N and Tsubura A: Trichilemmoma: An
immunohistochemical study of cytokeratins. Br J Dermatol.
149:99–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurokawa I, Takahashi K, Moll I and Moll
R: Expression of keratins in cutaneous epithelial tumors and
related disorders - distribution and clinical significance. Exp
Dermatol. 20:217–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blobel GA, Moll R, Franke WW and
Vogt-Moykopf I: Cytokeratins in normal lung and lung carcinomas. I.
Adenocarcinomas, squamous cell carcinomas and cultured cell lines.
Virchows Arch B Cell Pathol Incl Mol Pathol. 45:407–429. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Markey AC, Lane EB, Churchill LJ,
MacDonald DM and Leigh IM: Expression of simple epithelial keratins
8 and 18 in epidermal neoplasia. J Invest Dermatol. 97:763–770.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Onishi M, Yoshida A, Maeda F, et al: Two
cases of eccrine porocarcinonma with high level of serum CYFRA.
Skin Cancer. 29:38–42. 2014. View Article : Google Scholar
|