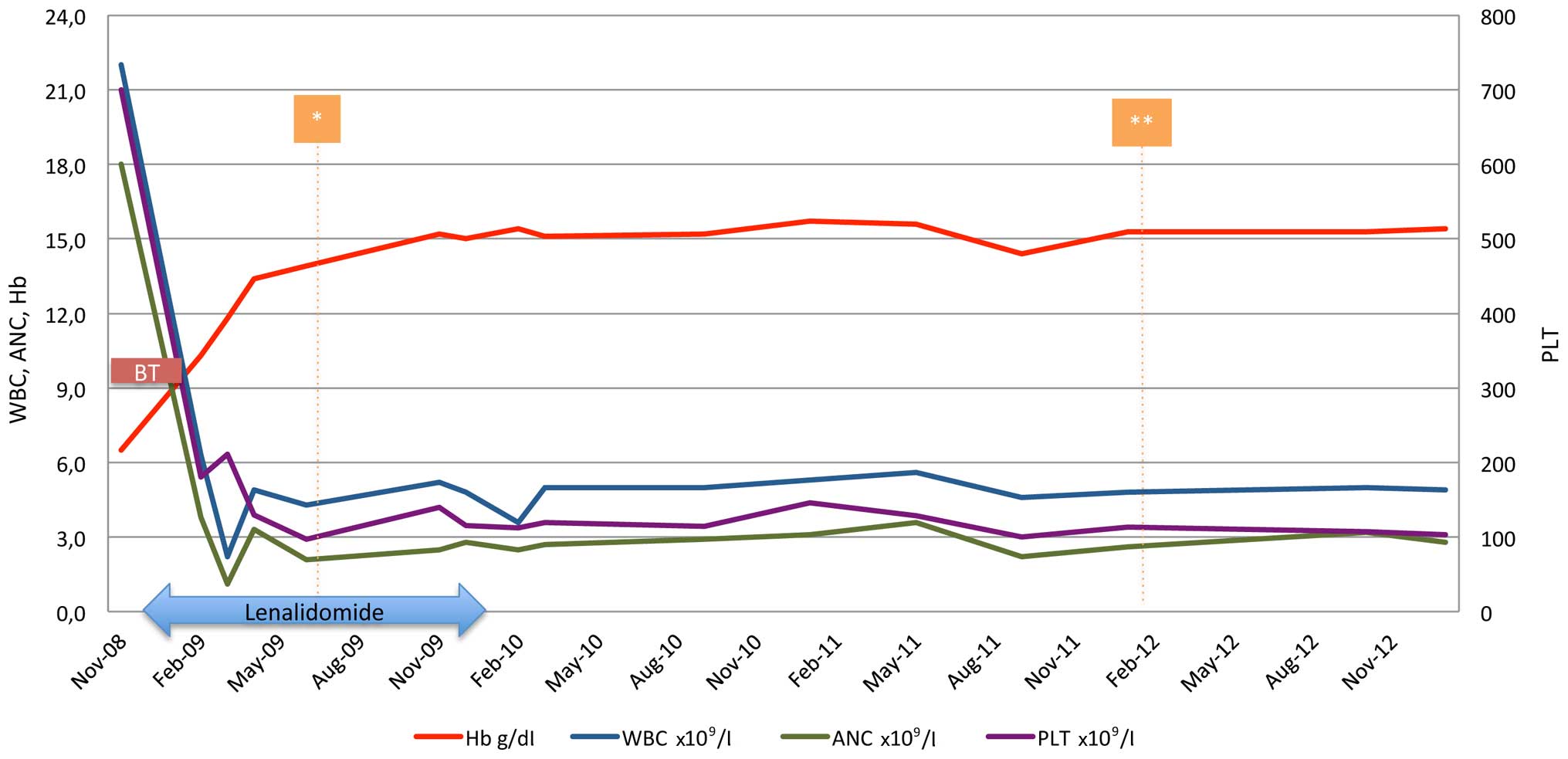
|
1
|
Bejar R, Levine R and Ebert BL: Unraveling
the molecular pathophysiology of myelodysplastic syndromes. J Clin
Oncol. 29:504–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cazzola M: Myelodysplastic syndrome with
isolated 5q deletion (5q-syndrome). A clonal stem cell disorder
characterized by defective ribosome biogenesis. Haematologica.
93:967–972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
List A, Dewald G, Bennett J, Giagounidis
A, Raza A, Feldman E, Powell B, Greenberg P, Thomas D, Stone R, et
al: Lenalidomide in the myelodysplastic syndrome with chromosome 5q
deletion. N Engl J Med. 355:1456–1465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baxter EJ, Scott LM, Campbell PJ, East C,
Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N,
et al: Acquired mutation of the tyrosine kinase JAK2 in human
myeloproliferative disorders. Lancet. 365:1054–1061. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scott LM, Campbell PJ, Baxter EJ, Todd T,
Stephens P, Edkins S, Wooster R, Stratton MR, Futreal PA and Green
AR: The V617F JAK2 mutation is uncommon in cancers and in myeloid
malignancies other than the classic myeloproliferative disorders.
Blood. 106:2920–2921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ingram W, Lea NC, Cervera J, Germing U,
Fenaux P, Cassinat B, Kiladjian JJ, Varkonyi J, Antunovic P,
Westwood NB, et al: The JAK2 V617F mutation identifies a subgroup
of MDS patients with isolated deletion 5q and a proliferative bone
marrow. Leukemia. 20:1319–1321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. IARC Press. Lyon,
France: 2008.
|
|
8
|
Greenberg P, Cox C, LeBeau MM, Fenaux P,
Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, et al:
International scoring system for evaluating prognosis in
myelodysplastic syndromes. Blood. 89:2079–2088. 1997.PubMed/NCBI
|
|
9
|
Azaceta G, Calasanz MJ, Dourdil V,
Bonafonte E, Izquierdo I and Palomera L: Response to lenalidomide
in a patient with myelodysplastic syndrome with isolated del(5q)
and JAK2 V617F mutation. Leuk Lymphoma. 51:1941–1943. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nomdedeu M, Maffioli M, Calvo X,
Martínez-Trillos A, Baumann T, Díaz-Beyá M, Aguilar JL, Rozman M,
Costa D, Esteve J, et al: Efficacy of lenalidomide in a patient
with myelodysplastic syndrome with isolated del(5q) and JAK2V617F
mutation. Leuk Res. 35:1276–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Musto P, Simeon V, Guariglia R, Bianchino
G, Grieco V, Nozza F, La Rocca F, Marziano G, Lalinga AV, Fabiani
E, et al: Myelodysplastic disorders carrying both isolated del(5q)
and JAK2 (V617F) mutation: Concise review, with focus on
lenalidomide therapy. Onco Targets Ther. 7:1043–1050.
2014.PubMed/NCBI
|
|
12
|
Sokol L, Caceres G, Rocha K, Stockero KJ,
Dewald DW and List AF: JAK2 (V617F) mutation in myelodysplastic
syndrome (MDS) with del(5q) arises in genetically discordant
clones. Leuk Res. 34:821–823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giagounidis A, Fenaux P, Mufti GJ, Muus P,
Platzbecker U, Sanz G, Cripe L, Von Lilienfeld-Toal M and Wells RA:
Practical recommendations on the use of lenalidomide in the
management of myelodysplastic syndromes. Ann Hematol. 87:345–352.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adès L, Le Bras F, Sebert M, Kelaidi C,
Lamy T, Dreyfus F, Eclache V, Delaunay J, Bouscary D, Visanica S,
et al: Treatment with lenalidomide does not appear to increase the
risk of progression in lower risk myelodysplastic syndromes with 5q
deletion. A comparative analysis by the groupe francophone des
myelodysplasies. Haematologica. 97:213–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pisani F, Orlandi G and Merola R:
Long-term response in a patient with del(5q) myelodysplastic
syndrome who discontinued lenalidomide and obtained a good response
and tolerance to rechallenge. Case Rep Oncol. 7:277–284. 2014.
View Article : Google Scholar : PubMed/NCBI
|