Introduction
Melanoma is a malignant tumor type which is highly
invasive and of metastatic character. These features depend on
alterations in the extracellular matrix (ECM) to modify cell
adhesion, migration and invasion.
The proteins laminin and type-IV collagen are major
structural constituents of the basement membrane, and serve as
promoters of cell proliferation, differentiation, adhesion and
migration via integrins and other cell surface receptors (1–11).
Increased expression of laminin and type-IV (1–7) collagen
(6,7,9–11) has also been demonstrated in melanoma
cell lines with increased proliferation and metastatic potential
(1–11).
While the function of the serum levels of laminin as
biomarkers in patients with melanoma has been previously indicated
(8), the majority of available data
are from pre-clinical studies, and the concept remains to be
further investigated and established in the clinical setting.
The present study investigated the concentration of
circulating laminin and type-IV collagen in cutaneous melanoma
patients and healthy controls and identified their association with
patient prognosis, various clinical factors and responsiveness to
chemotherapy in order to investigate whether their potential use as
novel diagnostic or prognostic biomarkers.
Materials and methods
Patients
The cohort of the present study comprised 60
histologically confirmed cutaneous melanoma patients admitted to
the Institute of Oncology (Istanbul University, Istanbul, Turkey).
Patients with bi-dimensionally measurable disease without any
history of chemotherapy or radiotherapy were included in the study.
Staging was performed according to the staging system of the
American Joint Committee on Cancer. The medical history of each
patient was reviewed in detail; furthermore, physical examination
and blood analyses were performed prior to the study. Patients with
an Eastern Cooperative Oncology Group performance status of ≤2 and
suitable blood parameters received various standard immunotherapy
or chemotherapy schemes, including interferon alpha, cisplatin,
dacarbazine or temozolomide, as well as novel agents, including
ipilimumab and vemurafenib based on the stage of the disease. The
revised Response Evaluation Criteria in Solid Tumours version 1.1
were used for assessing the patients' responsiveness to
chemotherapy.
For comparison, 30 age- and gender-matched healthy
subjects were included in the study. Informed consent was obtained
from all patients and the study was reviewed and approved by the
local Ethics Committee.
Measurement of serum laminin and
type-IV collagen levels
Serum samples were drawn from patients and healthy
controls by venipuncture and clotted at room temperature on first
admission prior to treatment. The sera were collected after
centrifugation and immediately frozen at −20°C until analysis.
Laminin ELISA (USCN Life Science Inc., Wuhan, China)
and Collagen Type IV ELISA (USCN Life Science Inc.),
double-antibody sandwich ELISA, were used for determination of the
serum levels of laminin and type-IV collagen.
Statistical analysis
Values are expressed as median values and their
ranges, standard deviation or standard error as indicated.
Mann-Whitney U and Kruskal-Wallis tests were used to compare
clinical and laboratory parameters to serum levels of laminin and
type-IV collagen. The Kaplan-Meier test was used to estimate the
survival and the differences in outcome were evaluated using the
log-rank test. P≤0.05 was considered to indicate a significant
difference between values. SPSS 16.0 software (SPSS Inc., Chicago,
IL, USA) was used for all statistical analyses.
Results
Patient characteristics
A total of 60 cutaneous melanoma patients were
enrolled in this study. Of these, 13 were classified as stage I–II,
14 as stage III and 31 as stage IV, while the stage remained
elusive for 2 patients (Table I). The
median age of the patients was 53.5 years (range, 16–88 years).
Patient characteristics and pathological features are listed in
Table I.
 | Table I.Patient characteristics and
pathology. |
Table I.
Patient characteristics and
pathology.
| Characteristics | n |
|---|
| Number of
patients | 60 |
| Age, years |
|
|
<50/≥50 | 26/34 |
| Gender |
|
|
Male/female | 33/27 |
| Localization of
lesion |
|
|
Axial/extremity/unknown | 38/16/6 |
| Histopathology |
|
|
Nodular/non-nodular/unknown | 9/31/20 |
| Stage of disease |
|
|
I–II/III/IV/unknown | 13/14/31/2 |
| Tumor
stagea |
|
|
T1–2/T3–4/unknown | 10/16/1 |
| Lymph node
statusa |
|
|
Negative/positive | 12/14 |
| M1 stage |
|
|
M1a+b/M1c | 13/18 |
| Serum hemoglobin
levels |
|
|
Low/normal/unknown | 19/40/1 |
| Serum white blood
cell count |
|
|
Normal/elevated/unknown | 50/9/1 |
| Serum lactate
dehydrogenase levels |
|
|
Normal/elevated | 48/12 |
| Erythrocyte
sedimentation rate |
|
|
Normal/elevated/unknown | 28/20/12 |
| Responsiveness to
chemotherapyb |
|
|
Yes/no/unknown | 10/11/10 |
Comparison of serum laminin and
type-IV collagen between melanoma patients and healthy
controls
There was no statistically significant difference in
the baseline serum levels of laminin between cutaneous melanoma
patients and healthy controls (P=0.45) (Table II). However, the baseline serum
levels of type-IV collagen of the melanoma patients were
significantly elevated compared with those in the control group
(P<0.001) (Table II). Clinical
parameters, including patient age, gender, localization of lesion,
histopathology, stage of disease and serum lactate dehydrogenase
(LDH) concentration were found to not be linked with the serum
levels of laminin, type-IV collagen or responsiveness to
chemotherapy (P>0.05) (Table
III).
 | Table II.Serum concentrations of laminin and
type-IV collagen in melanoma patients and healthy controls
determined by ELISA. |
Table II.
Serum concentrations of laminin and
type-IV collagen in melanoma patients and healthy controls
determined by ELISA.
|
| Patients (n=60) | Controls (n=30) |
|
|---|
|
|
|
|
|
|---|
| Parameters | Median | Range | Median | Range | P-value |
|---|
| Laminin (pg/ml) | 920.5 | 390.0–3,600.0 | 778.5 | 221.0–1,560.0 | 0.45 |
| Type-IV collagen
(ng/ml) | 54.5 | 27.0–123.0 | 10.5 | 3.0–20.0 | <0.001 |
 | Table III.Association of laminin and type-IV
collagen levels with patient characteristics. |
Table III.
Association of laminin and type-IV
collagen levels with patient characteristics.
|
| Laminin | Type-IV collagen |
|---|
|
|
|
|
|---|
| Characteristics | Median (range) | P-value | Median (range) | P-value |
|---|
| Age, years |
| 0.73 |
| 0.45 |
| ≥50 | 1008.0
(221–3,530) |
| 54.0 (13–123) |
|
|
<50 | 870.0
(470–3,600) |
| 54.5 (28–102) |
|
| Gender |
| 0.17 |
| 0.98 |
| Male | 921.0
(390–3,600) |
| 54.0 (28–123) |
|
|
Female | 860.0
(409–3,530) |
| 55.0 (27–115) |
|
| Localization of
lesion |
| 0.87 |
| 0.35 |
|
Axial | 919.0
(390–3,600) |
| 54.0 (27–123) |
|
|
Extremity | 891.0
(409–3,530) |
| 56.5 (35–86) |
|
| Histopathology |
| 0.32 |
| 0.32 |
|
Non-nodular | 843.0
(390–3,530) |
| 54.0 (28–102) |
|
|
Nodular | 1008.0
(521–3,600) |
| 64.0 (44–85) |
|
| Stage of disease |
| 0.96 |
| 0.60 |
| I–II | 1010.0
(390–1,557) |
| 54.0 (28–90) |
|
| III | 881.0
(409–2,650) |
| 54.0 (36–86) |
|
| IV | 920.0
(470–3,600) |
| 58.0 (27–123) |
|
| Tumor stage |
| 0.09 |
| 0.34 |
| T1–2 | 776.5
(390–1,375) |
| 53.5 (28–90) |
|
| T3–4 | 1054.0
(409–2,650) |
| 54.5 (36–86) |
|
| Lymph node status (in
M0 disease) |
| 0.46 |
| 0.71 |
|
Negative | 1061.0
(390–1,557) |
| 54.5 (28–90) |
|
|
Positive | 881.0
(409–2,650) |
| 54.0 (36–86) |
|
| Metastasis status (in
all patients) |
| 0.87 |
| 0.33 |
| 0 | 921.0
(390–2,650) |
| 54.0 (28–90) |
|
| 1 | 920.0
(470–3,600) |
| 58.0 (27–123) |
|
| M1 stage |
| 0.49 |
| 0.13 |
| a-b | 960.0
(470–3,530) |
| 45.0 (30–80) |
|
| c | 812.0
(521–3,600) |
| 62.0 (27–123) |
|
| Serum hemoglobin
levels |
| 0.62 |
| 0.23 |
|
Normal | 918.5
(390–3,600) |
| 53.5 (28–100) |
|
| Low | 980.0
(560–3,530) |
| 60.0 (35–123) |
|
| Serum white blood
cell count |
| 0.45 |
| 0.87 |
|
Normal | 921.0
(390–3,530) |
| 54.5 (28–115) |
|
|
Elevated | 816.0
(409–3,600) |
| 56.0 (28–123) |
|
| Erythrocyte
sedimentation rate |
| 0.63 |
| 0.83 |
|
Normal | 911.5
(390–2,690) |
| 54.0 (36–90) |
|
|
Elevated | 964.5
(470–3,530) |
| 55.0 (27–123) |
|
| Serum lactate
dehydrogenase level |
| 0.41 |
| 0.93 |
|
Normal | 918.5
(390–3,600) |
| 54.5 (28–102) |
|
|
Elevated | 1090.0
(521–3,530) |
| 52.0 (27–123) |
|
| Chemotherapy
responsivenessa |
| 0.47 |
| 0.09 |
|
Yes | 1005.0
(562–3,600) |
| 62.5 (36–102) |
|
| No | 780.0
(470–1,896) |
| 45.0 (27–100) |
|
Survival analysis
The median survival time of the patients was 26.0
months [95% confidence interval (CI): 21–30]. The one- and
three-year overall survival rates were 76.3% (95% CI: 64–88) and
51.0% (95% CI: 33–69), respectively. Survival was significantly
decreased for patients with distant metastasis (M1) (P<0.001),
advanced metastatic disease (M1c) (P=0.007), anemia (P=0.05),
elevated erythrocyte sedimentation rate (ESR) (P<0.001),
elevated serum LDH concentration (P<0.001) and no responsiveness
to chemotherapy (P=0.01) (Table IV).
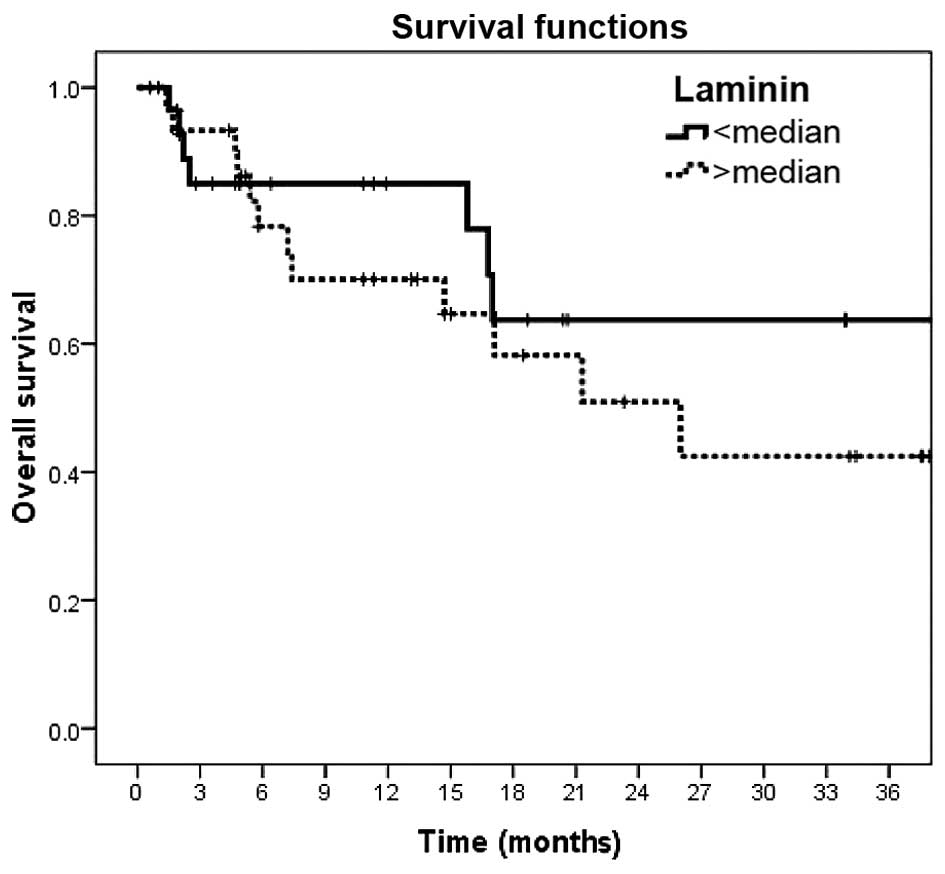
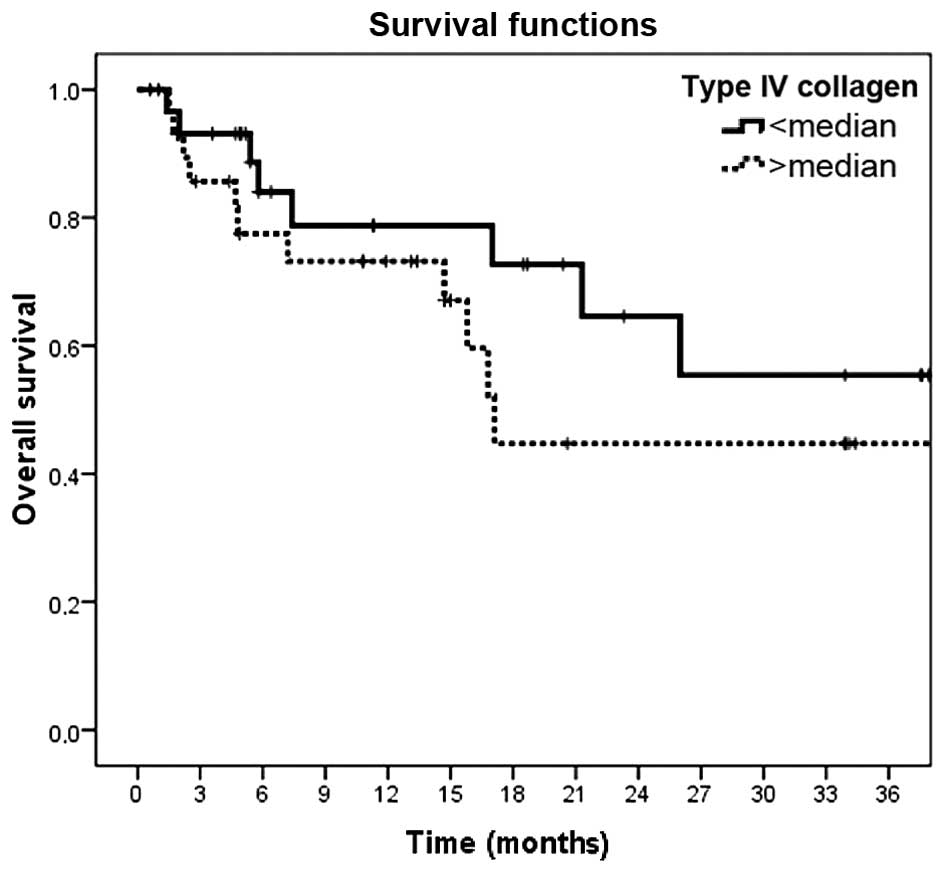
However, serum laminin and type-IV collagen levels were not found
to be of prognostic value in melanoma patients (P=0.36 and P=0.26,
respectively) (Table IV and Figs. 1 and 2).
 | Table IV.Univariate analysis of survival. |
Table IV.
Univariate analysis of survival.
|
Characteristics | Median survival
time (±SE) (months) | Two-year survival
rate (±SD) (%) | P-value |
|---|
| Age, years |
|
| 0.20 |
|
<50 | 27.3 (3.2) | 60.2 (12.2) |
|
|
≥50 | 24.4 (3.3) | 53.1 (11.6) |
|
| Gender |
|
| 0.35 |
|
Male | 25.1 (3.1) | 58.5 (10.1) |
|
|
Female | 25.7 (3.5) | 52.3 (14.0) |
|
| Localization of
lesion |
|
| 0.35 |
|
Axial | 27.1 (2.8) | 61.7 (9.7) |
|
|
Extremity | 25.9 (4.9) | 57.4 (17.1) |
|
| Histopathology |
|
| 0.50 |
|
Non-nodular | 29.6 (2.8) | 70.4 (9.7) |
|
|
Nodular | 23.0 (4.3) | 58.3 (25.1) |
|
| Tumor stage |
|
| 0.61 |
|
T1–2 | 35.6 (2.3) | 88.9 (10.5) |
|
|
T3–4 | 34.3 (3.4) | 66.7 (27.2) |
|
| Lymph node
status |
|
| 0.48 |
|
Negative | NR | 100.0 (0) |
|
|
Positive | NR | 90.9 (8.7) |
|
| Distant
metastasis |
|
| <0.001 |
| No | 36.8 (1.7) | 93.8 (6.1) |
|
|
Yes | 12.0 (2.3) | 9.9 (8.5) |
|
| M1 stage |
|
| 0.007 |
|
a-b | 18.3 (4.2) | 31.7 (18.0) |
|
| c | 7.7
(2.1) | NR |
|
| Serum hemoglobin
levels |
|
| 0.05 |
|
Normal | 29.4 (2.6) | 71.7 (8.4) |
|
|
Low | 17.9 (3.3) | 24.0 (14.4) |
|
| Serum white blood
cell count |
|
| 0.59 |
|
Normal | 26.4 (2.5) | 57.0 (9.0) |
|
|
Elevated | 14.5 (2.5) | NR |
|
| Erythrocyte
sedimentation rate |
|
| <0.001 |
|
Normal | 30.9 (2.5) | 71.1 (11.2) |
|
|
Elevated | 14.1 (3.7) | 21.4 (12.5) |
|
| Serum lactate
dehydrogenase levels |
|
| <0.001 |
|
Normal | 30.0 (2.3) | 66.4 (9.3) |
|
|
Elevated | 4.6 (0.8) | NR |
|
| Chemotherapy
responsivenessa |
|
| 0.01 |
|
Yes | 17.6 (3.9) | 27.4 (16.2) |
|
| No | 4.5
(0.7) | NR |
|
| Laminin |
|
| 0.36 |
| Low
(<median) | 28.9 (3.3) | 63.7 (11.8) |
|
| High
(>median) | 23.2 (3.0) | 50.9 (11.6) |
|
| Type-IV
collagen |
|
| 0.24 |
| Low
(<median) | 27.4 (3.1) | 64.6 (11.6) |
|
| High
(>median) | 23.1 (3.5) | 44.7 (12.4) |
|
Discussion
Laminin and type-IV collagen have been shown to be
present at the dermoepidermal junction and in the dermis
neighboring benign melanocytic nevi, dysplastic nevi, and in
melanoma (7). While a consistent loss
of laminin and type-IV collagen from the basement membrane zone has
been observed in areas adjacent to junctional melanocytic
proliferation, this decrease cannot sufficiently distinguish
between benign junctional proliferation and invasive early
melanoma. The type of staining in the surrounding area of deeper
invasive cells and intradermal nevus cells was similar and
suggested that melanocytic cells at these sites may actually
synthesize laminin and type-IV collagen (7).
Laminin, a major structural component of the
basement membrane, drives cell proliferation, differentiation,
adhesion and migration via integrins and other cell surface
receptors (1–8). Increased laminin expression was also
observed in melanoma cell lines and led to increased melanoma cell
proliferation and metastatic potential (1–7). Similar
to laminin, type-IV collagen is also an important structural
component of the ECM and promotes melanoma cell adhesion, migration
and invasion (6,7,9–11). It has been proposed that type-IV
collagen expression ensures the development of scaffolding required
for angiogenesis in melanoma (6,7,9–11). Type-IV
collagen is a ECM protein and is highly common within the basement
lamina and intima underlining the endothelium, excited chemotaxis
of a number of human melanoma cell lines by engaging α2β1 integrin
in vitro. Certain signal transduction incidents are driven
by type-IV collagen through β1 integrins, suggesting a significant
role for NF-κB in arranging chemotaxis of melanoma (9).
The majority of the findings on the abovementioned
ECM proteins have been obtained from pre-clinical tissue-cell
investigations (1–7,9–11). Apart from the present study, laminin
and type-VI collagen have been assessed in the sera of melanoma
patients in only one other study by Burchardt et al
(8), who examined the serum levels of
laminin, type-VI collagen, hyaluronan and tenascin-C in the sera of
6 patients with stage-I/II cutaneous melanoma and 6 patients with
metastatic disease via ELISA. In addition, four melanoma cell lines
were also analyzed for the expression of these markers. No
differences in laminin serum concentrations were observed between
healthy individuals and stage I/II melanoma patients or between
stage I/II melanoma patients and those with metastatic disease.
However, compared to those in healthy controls, serum laminin
levels were significantly elevated in metastatic patients
(P=0.018). Furthermore, the serum levels of type-VI collagen in
stage I/II (P=0.004) and IV melanoma patients (P=0.007) were
elevated compared to those in healthy controls. No significant
difference was detected between stage I/II and IV patients. The
authors suggested that these ECM markers were involved in melanoma
growth and extracellular matrix remodeling in the course of
melanoma progression (8). These
findings indicated that collagen type-VI and laminin may serve as
novel serum markers for melanoma progression.
In the present study, a total of 60 patients various
clinical stages of melanoma were enrolled. Similar to the study by
Burchardt et al (8), the serum
concentrations of these markers were quantitatively analyzed by
solid-phase ELISA. In the present study, the baseline serum levels
of type-IV collagen were significantly elevated in melanoma
patients compared to those in the control group (P<0.001).
However, no statistically significant difference in the baseline
serum levels of laminin were detected between melanoma patients and
healthy controls. Furthermore, clinical parameters, including
patient age, gender, localization of lesion, histopathology,
clinical stage of disease, serum LDH concentration and
responsiveness to chemotherapy were found not to be associated with
the serum levels of laminin and type-IV collagen. In addition, the
serum levels of the two ECM proteins were shown to not be
associated with patient survival and to therefore not to possess
any prognostic value for melanoma patients.
In conclusion, the present study indicated that the
serum levels of type-IV collagen may serve as a diagnostic
biomarker in melanoma patients, while laminin levels were of no
diagnostic value. However, the serum levels of neither of the two
ECM proteins had any predictive or prognostic value in melanoma
patients. The small size of the patient cohort and the short time
to follow-up of the present study (36 months) represent significant
limitations and may have affected the results. However, the present
study contributed to the current understanding of the roles of
these ECM proteins in the pathology of cutaneous melanoma in an
anatolian or Asia minor population, and patients at various stages
were included. Studies on larger patient populations are required
to further determine the exact role of these biomarkers in
cutaneous melanoma patients.
References
|
1
|
Chen HB, Chen L, Zhung JK, Chow VW, Wu BQ,
Wang ZH, Cheng SB and Chew EC: Expression of laminin in metastatic
melanoma cell lines with different metastatic potential. Anticancer
Res. 21:505–508. 2001.PubMed/NCBI
|
|
2
|
Mortarini R, Gismondi A, Maggioni A,
Santoni A, Herlyn M and Anichini A: Mitogenic activity of laminin
on human melanoma and melanocytes: Different signal requirements
and role of beta 1 integrins. Cancer Res. 55:4702–4710.
1995.PubMed/NCBI
|
|
3
|
Oikawa Y, Hansson J, Sasaki T, Rousselle
P, Domogatskaya A, Rodin S, Tryggvason K and Patarroyo M: Melanoma
cells produce multiple isoforms and strongly migrate on α5
laminin(s) via several integrin receptors. Exp Cell Res.
317:1119–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lugassy C, Torres-Muñoz JE, Kleinman HK,
Ghanem G, Vernon S and Barnhill RL: Overexpression of
malignancy-associated laminins and laminin receptors by angiotropic
human melanoma cells in a chick chorioallontoic membrane model. J
Cutan Pathol. 36:1237–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gradilone A, Gazzaniga P, Cigna E, et al:
Fibronectin and laminin expression in sentinel lymph nodes of
patients with malignant melanoma. Br J Dermatol. 157:398–401. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramos DM, Berston ED and Kramer RH:
Analysis of integrin receptors for laminin and type IV collagen on
metastatic B16 melanoma cells. Cancer Res. 50:728–734.
1990.PubMed/NCBI
|
|
7
|
Mackie RM, Clelland DB and Skerrow CJ:
Type IV collagen and laminin staining patterns in benign and
malignant cutaneous lesions. J Clin Pathol. 42:1173–1177. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burchardt ER, Hein R and Bosserhoff AK:
Laminin, hyaluronan, tenascin-C and type VI collagen levels in sera
from patients with malignant melanoma. Clin Exp Dermatol.
28:515–520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hodgson L, Henderson AJ and Dong C:
Melanoma cell migration to type IV collagen requires activation of
NF-kappaB. Oncogene. 22:98–108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pasco S, Brassart B, Ramont L, et al:
Control of melanoma cell invasion by type IV collagen. Cancer
Detect Prev. 29:260–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daniels KJ, Boldt HC, Martin JA, et al:
Expression of type IV collagen in uveal melanoma: Its role in
pattern formation and tumor progression. Lab Invest. 75:55–66.
1996.PubMed/NCBI
|