Introduction
Mesenteric leiomyosarcoma is a rare disease with
poor prognosis. Since 1998, it is acknowledged that the proper
diagnosis of leiomyosarcoma requires immunohistochemical (IHC)
analysis to differentiate it from gastrointestinal stromal tumor
(GIST) (1), which is the most common
mesenchymal tumor of the gastrointestinal tract. Several GISTs
diagnosed before 1998 were misclassified as leiomyosarcomas;
therefore, the frequency of leiomyosarcoma may have been previously
overestimated. The clinical and pathological characteristics of
mesenteric leiomyosarcoma have not been clearly determined. In this
report, we present the case of a patient who underwent curative
surgery for leiomyosarcoma arising in the descending mesocolon and
for a metachronous liver metastasis, together with the
clinicopathological findings and a literature review.
Case report
A 76-year-old woman noticed a mass in her left upper
abdomen and consulted her local clinic, where a 12-cm abdominal
tumor was detected by ultrasonographic evaluation. The patient was
referred to the Saitama Medical Center, Jichi Medical University
(Saitama, Japan) for further investigation and treatment. The
patient's past medical history included hepatitis C infection, an
ovarian cyst and appendicitis. The cyst and the appendix were
surgically resected at the age of 4 and 34 years, respectively. The
patient had a family history of cancer, as her mother had developed
colorectal cancer. Physical examination revealed a palpable mass,
sized ~5 cm, in the left upper abdomen, which was elastic hard and
movable. No lymph nodes were palpable. The patient did not suffer
from nausea. The tumor markers were marginally elevated
(carcinoembryonic antigen, 5.6 ng/ml and carbohydrate antigen 19-9,
22.2 U/ml). Interleukin-2 receptor level was within the normal
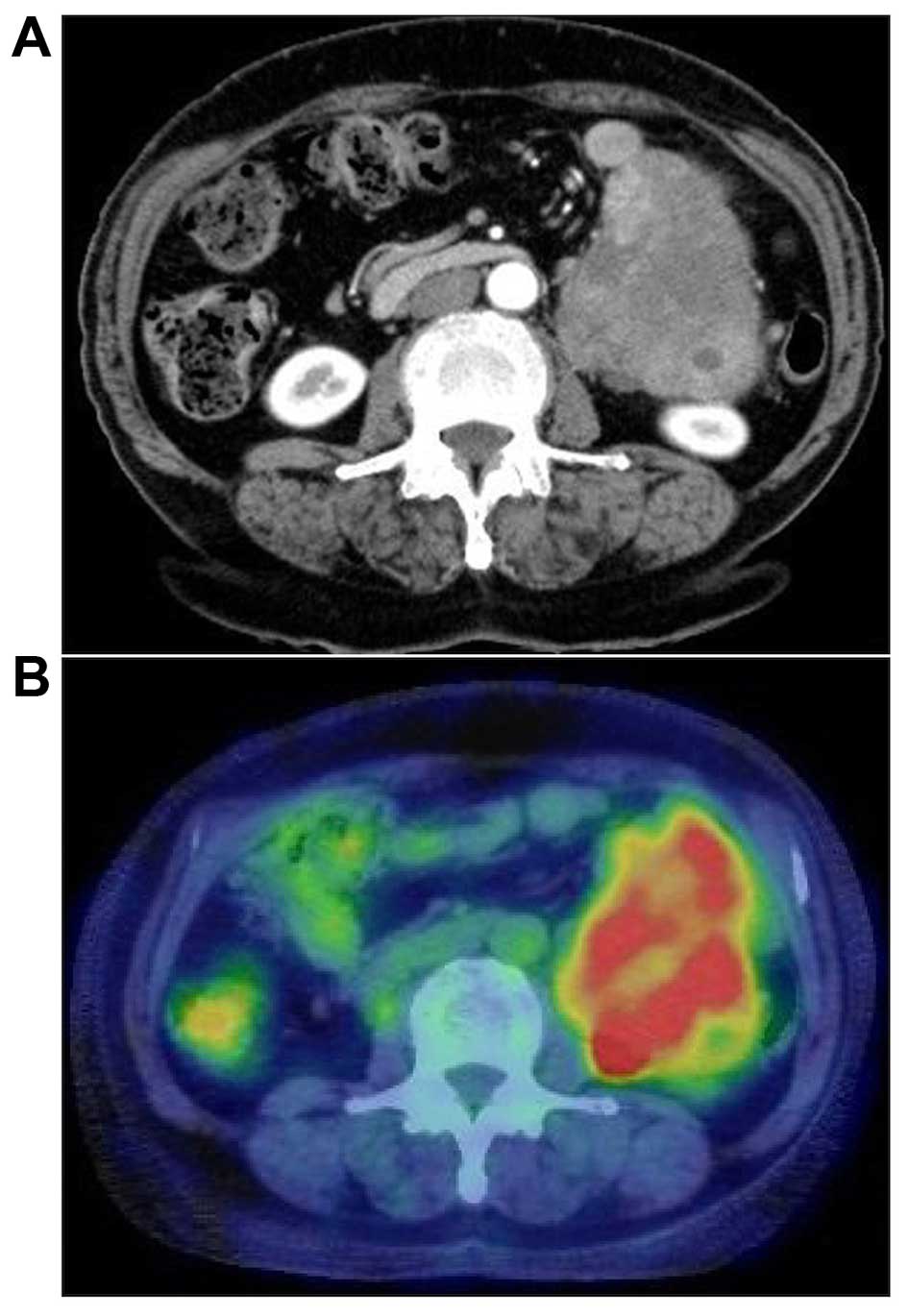
range. Abdominal computed tomography (CT) revealed a 13-cm tumor,
heterogeneously enhanced, located in the abdominal cavity between
the tail of the pancreas and the anterior surface of the left
kidney. The margin with the surrounding organs was clear, except
for the small intestine. There was no evidence of ascites, liver
metastasis, or enlarged lymph nodes (Fig.
1A). The tumor was fluorodeoxyglucose (FDG)-avid on
FDG-positron emission tomography (PET)/CT (Fig. 1B). Colonoscopy revealed no evidence of
a tumor in the descending colon. The patient was diagnosed with a
tumor arising in the retroperitoneal or descending mesocolon and
underwent surgery in June, 2012. Intraoperatively, the tumor was
located in the descending mesocolon, it was movable and easily
excised from the left psoas muscle and urinary duct. However, the
tumor invaded the mesenteric vein; therefore, en bloc removal of
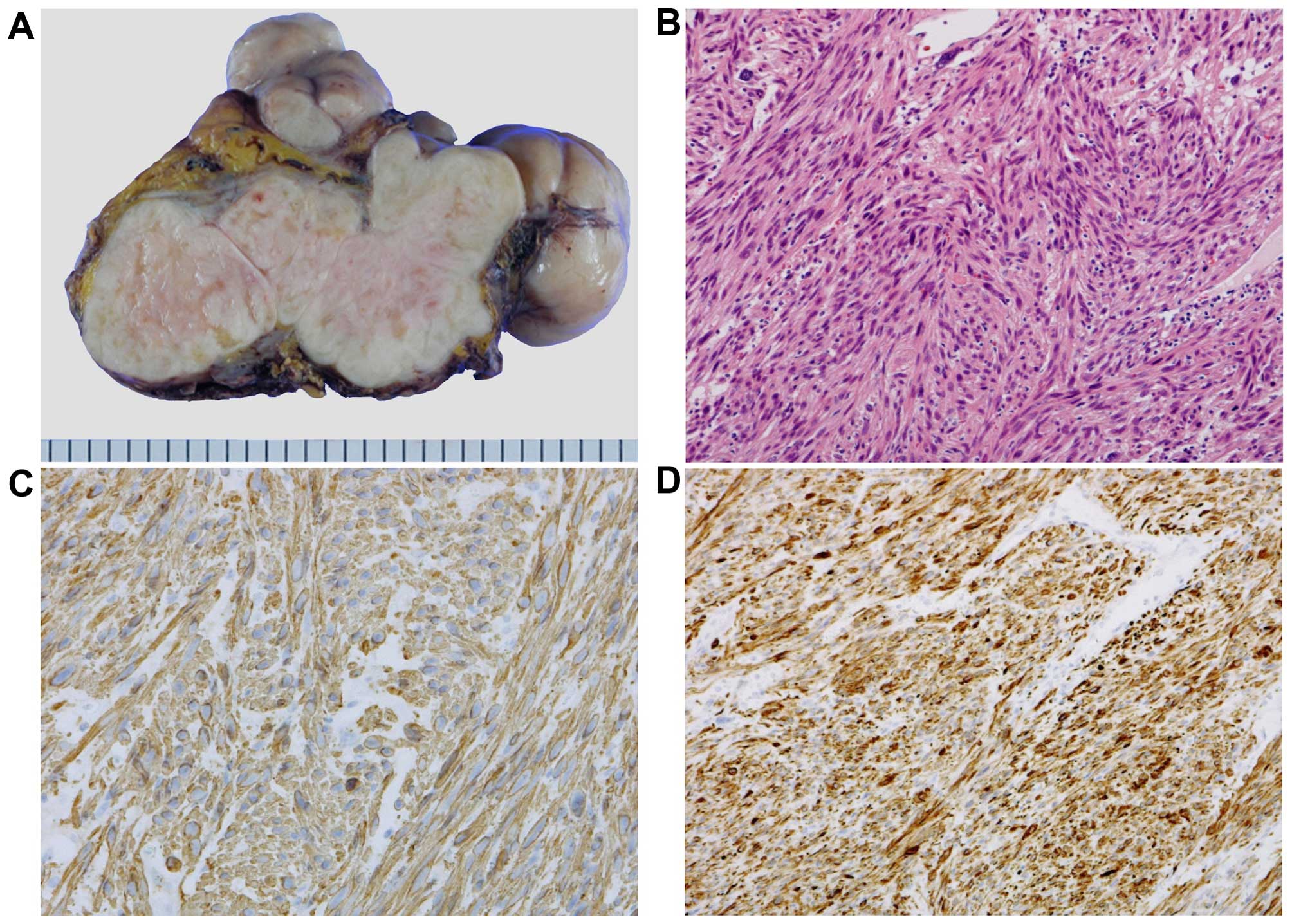
the descending colon was performed. Macroscopically, the size of
the tumor was 14×9×9 cm, it weighed 500 g, its surface was smooth
and elastic hard, and the cut surface was solid and white (Fig. 2A). The histological findings included
high cellularity, spindle-shaped cells with blunted hyperchromatic
nuclei, exhibiting a trabecular proliferation pattern that
indicated differentiation towards smooth muscle (Fig. 2B). IHC analysis revealed that the
tumor was positive for smooth muscle actin and desmin (Fig. 2C and D) and negative for CD117 (c-KIT)
and S-100. From these findings, the final diagnosis was confirmed
as leiomyosarcoma of the descending mesocolon. The patient's
postoperative course was uncomplicated and she was discharged from
the hospital 11 days after surgery. She was followed up every 6
months by blood tests and imaging studies [CT, magnetic resonance
imaging (MRI) and ultrasonography] and did not receive any adjuvant
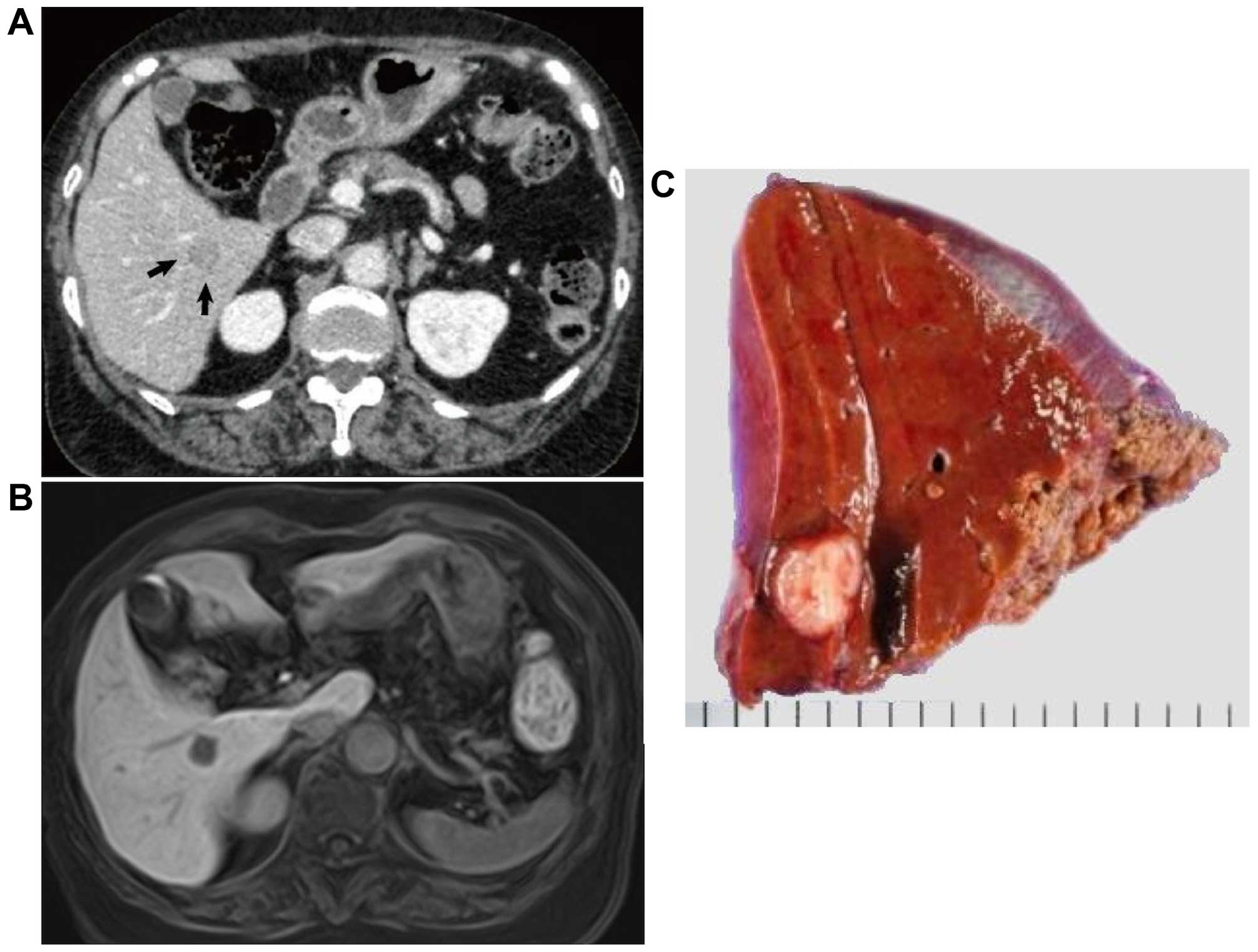
chemotherapy. A tumor at segment VI in the right lobe of the liver
was detected 24 months after the surgery. The tumor size was ~15 mm
and marginally enhanced on CT (Fig.
3A). The tumor was also detected as a hypointense lesion on
gadolinium ethoxybenzyl diethylenetriamine pentaacetic
acid-enhanced MRI with a T1-weighted image of the hepatobiliary
phase (Fig. 3B), but not detected on
FDGPET/CT. The patient underwent partial liver resection to remove
the tumor. The tumor was measured to be 12×10 mm (Fig. 3C) and was diagnosed as a metastatic
lesion from the leiomyosarcoma of the descending mesocolon by
pathological examination. At the most recent follow-up, 16 months
after the surgery for liver metastasis and 40 months after the
primary surgery, the patient remains disease-free.
Discussion
We herein report a case who underwent surgery for
primary leiomyosarcoma of the descending mesocolon and a single
metachronous liver metastasis. Mesenteric leiomyosarcoma is a rare
malignancy. The first mesenteric leiomyosarcoma case in the
literature was reported by Derechin et al (1) in 1956. Proper diagnosis of
leiomyosarcoma requires IHC to differentiate this type of lesion
from GIST, the most common mesenchymal tumor of the
gastrointestinal tract. However, older reports on mesenteric smooth
muscle tumors cannot be included in the current classification,
since, at the time, the studies did not differentiate between
leiomyosarcoma and GIST. Thus, several GISTs may have been
misclassified as leiomyosarcomas; therefore, the frequency of
leiomyosarcoma may have been previously overestimated. We searched
the PubMed database using the key words ‘leiomyosarcoma AND
(mesentery OR mesenteric)’, excluding articles that were published
prior to 1998, which was the year the GIST concept was introduced
(2). A total of 163 articles were
initially identified and we eliminated reports that did not apply
IHC for the differential diagnosis of GIST or reports that were not
written in English. Including our case, only 13 patients have been
reported to be diagnosed with mesenteric leiomyosarcoma (Table I). The median age of the patients was
62 years (range, 13–82 years) and they included 6 men and 7 women.
The most common site developing leiomyosarcoma was the mesentery of
the small intestine (6 cases) followed by the mesocolon (4 cases)
and mesorectum (1 case). The remaining 2 cases were described as
just mesentery, with no more specific location information. Of the
12 cases, 11 had follow-up information, with a median of 18 months
(range, 3–59 months). Surgical resection with a wide margin of
normal tissue is the most effective treatment strategy for
mesenteric leiomyosarcoma (3). All 13
cases received surgery for the primary lesion; 6 patients succumbed
to the disease during the follow-up period (3–58 months). No
patient was reported to survive for >59 months. Of the patients
who succumbed to the disease, 1 died with the originally diagnosed
leiomyosarcoma (no information on surgical intervention was
reported); 4 had developed liver metastasis, with (n=3) or without
(n=1) local recurrence after surgical resection of the primary
lesion; and 1 patient succumbed to lung metastasis after removal of
the primary lesion (Table I). Liver
is the most common organ in which metastatic lesions developed
among these cases. Of note, all the patients who were followed up
after surgery for >6 months developed liver metastasis
synchronously (n=2) or metachronously (n=6). One case reported by
Miettinen et al (4) developed
a liver metastasis at an undetermined time after the operation and
during the12-month follow-up. The median interval for developing
liver metastasis after primary surgery was 20 months (range, 0–48
months). The majority of these liver metastases were multiple, with
1 case developing metastases both in the liver and lung. Only 2
patients, including the one reported in this study, developed
single liver metastasis (5). Three
cases, including the patient reported in our study, underwent
surgery for liver metastasis (6), and
3 cases received chemotherapy for the metastatic lesions (5,7). There is
no mention of surgical intervention or chemotherapy treatment for
the secondary liver lesions in the remaining 3 cases. Some reports
demonstrated that doxorubicin-containing chemotherapy improved the
outcome of patients with metastatic leiomyosarcoma (8,9). Although
these reports did not include patients with mesenteric
leiomyosarcoma, due to its rarity, doxorubicin-containing
chemotherapy may also be effective in reducing the incidence of
metastasis or recurrence of mesenteric leiomyosarcoma. However, a
standardized chemotherapy regimen for metastasis or recurrence of
this type of tumor is not yet established. In our case, 40 months
after the initial removal of the primary lesion, the patient
remains disease-free. The favorable postoperative course over an
extended period of time was achieved by curative resection of both
the primary tumor and the metachronous liver metastasis, without
adjuvant chemotherapy. Albeit the current literature is limited due
to the low incidence of mesenteric leiomyosarcomas, the data reveal
a high incidence of liver metastasis in these patients. We
recommend periodic monitoring using imaging techniques following
surgical resection, as it would be highly beneficial in the
clinical management of mesenteric leiomyosarcoma patients,
facilitating early detection and curative resection of metachronous
liver metastases. Further studies are required to elucidate the
clinical and pathological characteristics of mesenteric
leiomyosarcoma patients.
 | Table I.Reported cases of mesenteric
leiomyosarcoma studied by IHC for differential diagnosis of
GIST. |
Table I.
Reported cases of mesenteric
leiomyosarcoma studied by IHC for differential diagnosis of
GIST.
| First author
(year) | Age/gender | Symptoms | Size (mm) | Location
(mesentery) | Operative
procedure | Outcome (months) | Cause of death | Distant
metastasis | Interval between
primary and metastatic lesion (months) | Chemotherapy | Refs. |
|---|
| Miettinen (1999) | 41/M | N.M. | N.M. | N.M. | N.M. | Alive (12) | – | Liver | N.M. | N.M. | (4) |
| Miettinen (1999) | 86/M | N.M. | N.M. | N.M. | N.M. | Dead (3) | Present disease | N.M. | – | N.M. | (4) |
| Fukanaga (2004) | 62/F | Mass | 140 | Sigmoid colon | Extirpation | Dead (10) | Liver metastasis | Liver | 0 | No | (10) |
| Simonovich
(2006) | 82/F | Mass, pain | 110 | Small intestine | Resection with small
intestine | Alive (24) | – | Liver | 24 | No | (3) |
| Iwasaki (2010) | 13/M | Mass | 100 | Small intestine | Resection with small
intestine | Alive (3) | – | No | – | – | (11) |
| Koczkowska
(2013) | 62/F | – | 78 | Small intestine | Extirpation | Dead (24) | Liver metastasis,
local recurrence | Liver, local | 0 | Yes | (7) |
| Koczkowska
(2013) | 46/F | Mass | N.M. | Sigmoid colon | Resection with colon
and uterus | Dead (58) | Liver metastasis,
local recurrence | Liver, local | 48 | Yes | (7) |
| Mizobe (2013) | 65/M | Mass, pain | 200 | Ascending colon | Ileocecal
resection | Dead (18) | Liver metastasis,
local recurrence | Liver, local | 11 | Yes | (5) |
| Dasgupta (2016) | 62/F | Fullness | 220 | Small intestine | Resection with small
intestine | Alive (6) | – | No | – | – | (12) |
| Sidhic (2015) | 33/M | Pain | 150 | Small intestine | Resection with small
intestine | Alive (0) | – | No | – | – | (13) |
| Hamed (2015) | 49/F | N.M. | N.M. | Small intestine | Resection with small
intestine | Alive (59) | – | Liver | 29 | N.M. | (6) |
| Hamed (2015) | 46/M | N.M. | N.M. | Rectum | Resection with
rectum | Dead (26) | Lung metastasis | Liver, lung | 16 | N.M. | (6) |
| Present case | 76/F | Mass | 140 | Descending colon | Resection with
colon | Alive (40) | – | Liver | 24 | No |
Glossary
Abbreviations
Abbreviations:
|
GIST
|
gastrointestinal stromal tumor
|
|
IHC
|
immunohistochemical
|
|
CT
|
computed tomography
|
|
FDGPET
|
fluorodeoxyglucose positron emission
tomography
|
|
MRI
|
magnetic resonance imaging
|
References
|
1
|
Derechin W and Wolfe S: Leiomyosarcoma of
the mesentery. Can Med Assoc J. 75:1028–1029. 1956.PubMed/NCBI
|
|
2
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simonovich CJ, Hardman JM, Navin JJ,
Jacobs J and Fergusson N: An unusual abdominal tumor -
leiomyosarcoma of the mesentery: A case report. Hawaii Med J.
65:18–20. 2006.PubMed/NCBI
|
|
4
|
Miettinen M, Monihan JM, Sarlomo-Rikala M,
Kovatich AJ, Carr NJ, Emory TS and Sobin LH: Gastrointestinal
stromal tumors/smooth muscle tumors (GISTs) primary in the omentum
and mesentery: clinicopathologic and immunohistochemical study of
26 cases. Am J Surg Pathol. 23:1109–1118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizobe T, Akagi Y, Ishikawa H, Shiratsuchi
I, Oka Y, Kinugasa T, Ohshima K, Setojima K and Shirouzu K:
Gemcitabine with paclitaxel therapy against mesocolic
leiomyosarcoma: A case report. Anticancer Res. 33:2929–2933.
2013.PubMed/NCBI
|
|
6
|
Hamed MO, Roberts KJ, Merchant W and Lodge
JP: Contemporary management and classification of hepatic
leiomyosarcoma. HPB (Oxford). 17:362–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koczkowska M, Lipska BS, Grzeszewska J,
Limon J, Biernat W and Jassem J: Primary leiomyosarcoma of the
mesentery in two sisters: Clinical and molecular characteristics.
Pol J Pathol. 64:59–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maki RG, Wathen JK, Patel SR, Priebat DA,
Okuno SH, Samuels B, Fanucchi M, Harmon DC, Schuetze SM, Reinke D,
et al: Randomized phase II study of gemcitabine and docetaxel
compared with gemcitabine alone in patients with metastatic soft
tissue sarcomas: Results of sarcoma alliance for research through
collaboration study 002 (corrected). J Clin Oncol. 25:2755–2763.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Penel N, Italiano A, Isambert N, Bompas E,
Bousquet G and Duffaud F: French Sarcoma Group (Groupe Sarcome
Français/Groupe d'Etude des Tumeurs Osseuses): Factors affecting
the outcome of patients with metastatic leiomyosarcoma treated with
doxorubicin-containing chemotherapy. Ann Oncol. 21:1361–1365. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukunaga M: Neuron-specific
enolase-producing leiomyosarcoma of the mesentery. APMIS.
112:105–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwasaki M, Kitaguchi K and Kobayashi H:
Mesenteric leiomyosarcoma in a 13-year-old boy. J Pediatr Surg.
45:1893–1895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dasgupta S, Chakrabarti S, Ghosh S and Das
S: Ileal Mesenteric Leiomyosarcoma-Report of a Rare Neoplasm. J
Gastrointest Cancer. 47:114–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sidhic AK, Ranjith M, Ali KP and Tej PR:
Leiomyosarcoma of the mesentry, a rare mesentric tumour. Int J Surg
Case Rep. 7C:58–60. 2015. View Article : Google Scholar : PubMed/NCBI
|