Introduction
Gastric cancer is the second leading cause of
cancer-associated mortality worldwide with a five-year survival
rate of <30% (1,2). For patients with localized gastric
cancer, surgery remains the basic treatment (3). However, the majority of patients are
diagnosed at an advanced stage, at which radical surgery is no
longer possible, and the outcome of available treatments remains
unsatisfactory. Palliative chemotherapy is widely applied for the
treatment of advanced gastric cancer patients and has become a
standard clinical practice (4).
Glycosylation is one of the biochemical mechanisms
which regulate cellular functions. Aberrant glycosylation of
numerous proteins has been identified in nearly all types of
cancers and has been confirmed to be associated with tumor
progression, metastasis and the survival rate of patients (5–8). Most
clinical tumor markers are glycoproteins; however, detection of
specific cancer-associated alterations in glycan structures, such
as alpha-fetoprotein-(AFP)L3 (9,10), may
improve their specificity; furthermore, novel biomarkers are
currently being discovered (11).
Tumor abnormal protein (TAP) is a collective term
for glycoproteins produced during the development of a variety of
malignant tumors as their common feature. Meezan et al
(12) first demonstrated that
cancer-associated glycans differ from glycans on healthy cells.
Numerous tumor-associated glycans are present at low levels in
normal tissues and at elevated levels on tumors (13). When TAP levels reach a certain
threshold, they can be detected in the peripheral blood.
The present study was performed to assess the
prognostic value of TAP in gastric cancer patients.
Materials and methods
Patients
A total of 42 patients with histological diagnosis
of gastric cancer who were treated at the Affiliated Changzhou
Tumor Hospital of Soochow University (Changzhou, China) between
January and June 2014 were enrolled in the present study. Written
informed consent was obtained from all the patients. All procedures
were performed according to the guidelines of the local Ethics
Committee. All patients selected for the present study had been
diagnosed with gastric cancer by histopathology and had not
received any pre-operative adjuvant chemotherapy, radiotherapy or
targeted therapy. Patients presenting with other types of malignant
tumor alongside gastric cancer, rheumatoid arthritis or active
tuberculosis, as well as immunocompromised, pregnant and diabetic
patients, were excluded from the study. The clinical data of the
patients are shown in Table I. In
addition, 56 healthy volunteers were recruited for the present
study. All the patients were followed up until July, 2015.
 | Table I.Clinical characteristics of the study
population (n=42). |
Table I.
Clinical characteristics of the study
population (n=42).
| Characteristics | Patient number | % |
|---|
| Age, years [median
(range)] | 66 (33–78) |
| Gender
(female/male) | 12/30 | 28.6/71.4 |
| Localization |
|
|
|
Cardia | 21 | 50.0 |
| Body | 11 | 26.2 |
|
Antrum | 10 | 23.8 |
| Tumor size, cm |
|
|
|
<5 | 15 | 35.7 |
| ≥5 | 27 | 64.3 |
| Invasion depth |
|
|
|
T1-T3 | 8 | 19.0 |
| T4 | 34 | 81.0 |
| Differentiation |
|
|
| Well | 14 | 33.3 |
| Poor | 28 | 66.7 |
| TNM stage |
|
|
| I–II | 14 | 33.3 |
| III | 7 | 16.7 |
| IV | 21 | 50.0 |
Detection of TAP
TAP was detected using a TAP testing kit and
examination system (Zhejiang Ruisheng Medical Technology, Ltd.,
Cixi, China). Peripheral blood (25 µl) was collected from the
fingertip of each patient and blood smears of uniform thickness
were prepared, followed by drying at ambient temperature in a
horizontal position for 10 min. Coagulation auxiliaries were then
added to the blood smears and after 1.5–2 h, condensed particles
had formed. The shape of these particles, which is indicative of
the TAP status of the sample, was then examined under a TAP
detection image analyzer. Samples were identified to be
TAP-positive when condensed particles had formed fulfilling the
following criteria: A diameter of >38 µm, with marginal
integrity, refraction of the oval, irregular or polygonal shape, a
lightly stained area in the center and accumulation of small
fragments in the immediate surrounding area of the particle.
Samples were confirmed as TAP-negative when dendritic-shaped or no
particles were observed.
Statistical analysis
Statistical analysis was performed using the
Statistical Package for the Social Sciences (SPSS) version 16.0
(SPSS Inc., Chicago, IL, USA). Data were compared by using the
χ2 test. Progression-free survival was defined as the
period from the time of diagnosis to disease progression or
succumbing to the disease. The Kaplan-Meier curve was used to
describe progression-free survival (PFS) and differences between
groups were compared by using the log-rank test. Univariate
analysis comprised gender (male vs. female), age (<60 vs. ≥60
years), differentiation (well vs. poor), tumor-node-metastasis
(TNM) stage (I–III vs. IV) and TAP status (positive vs. negative).
Factors with statistical significance or a tendency towards
significance (P<0.2) at univariate analysis were subjected to
multivariate Cox regression analysis. For all comparisons,
P<0.05 was considered to indicate a statistically significant
difference.
Results
A total of 42 gastric cancer patients (30 males and
12 females; age range, 33–78 years) were enrolled in the present
study, whose clinical characteristics are listed in Table I. TAP was detected in 27 gastric
cancer patients, accounting for a TAP-positive rate of 64.3%, which
was significantly higher than that in healthy volunteers (16.1%;
P<0.01) (Table II). As shown in
Table III, the prevalence of TAP
positivity was not associated with gender, tumor location, tumor
size, depth of invasion and the presence of lymph-node metastasis
(P>0.05), while it was significantly associated with the age,
TNM stage and degree of differentiation (P=0.008, 0.034 and 0.040,
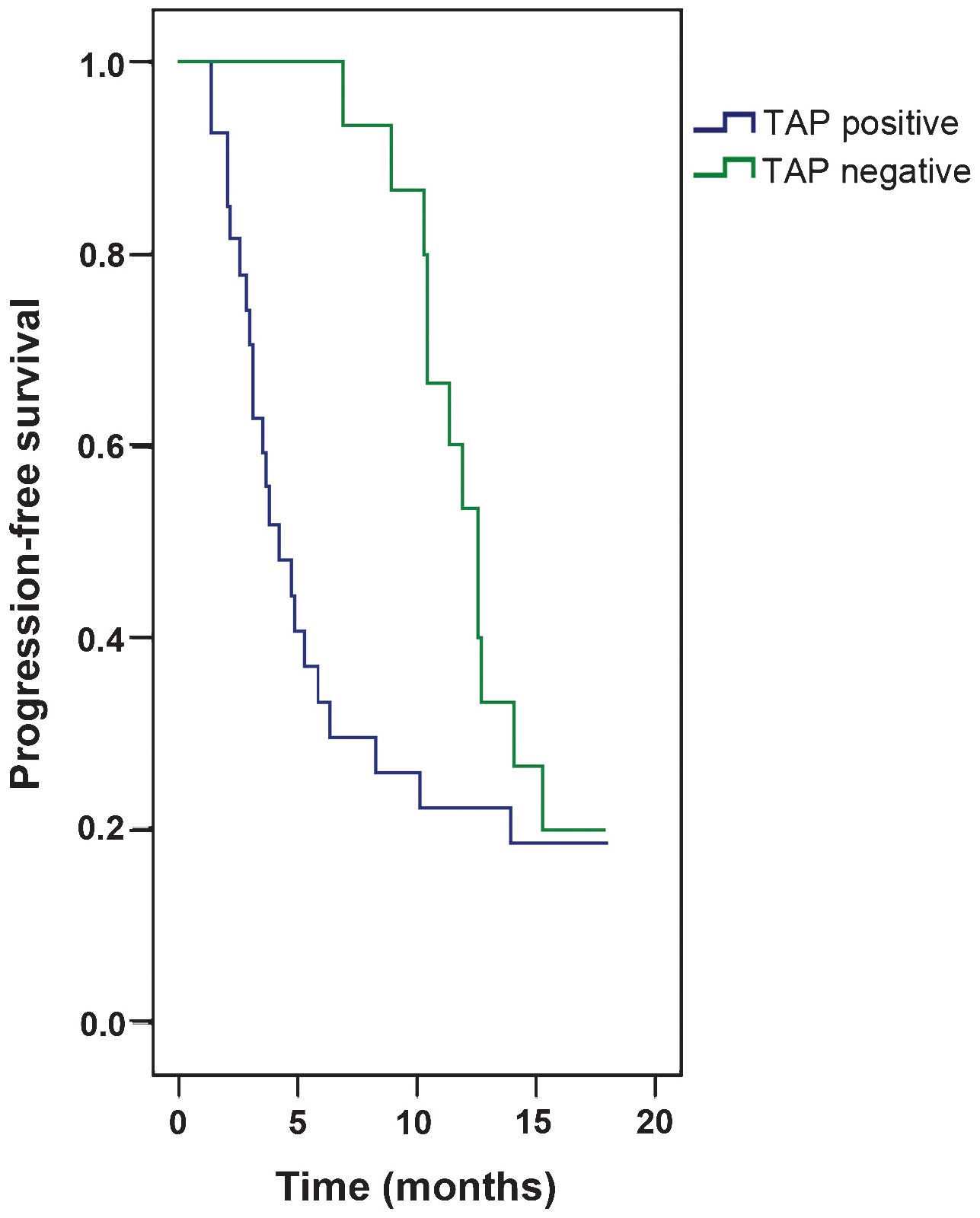
respectively). The median PFS of TAP-positive patients was 4.2
months, which was significantly lower than that for TAP-negative
patients (12.6 months; P=0.043). According to multivariate Cox
regression analysis, the TAP status (P<0.001; hazard ratio (HR),
64.487; 95% confidence interval (CI), 11.905–349.315), degree of
differentiation (P<0.001; HR, 17.279; 95% CI, 4.504–66.296) and
TNM stage (P<0.001; HR, 45.480; 95% CI, 9.370–220.758) were
independent predictive factors for reduced PFS (Table IV and Fig.
1).
 | Table II.TAP detection in gastric cancer
patients and healthy volunteers. |
Table II.
TAP detection in gastric cancer
patients and healthy volunteers.
| Groups | TAP-positive | TAP-negative | Positive rate
(%) | χ2 | P-value |
|---|
| Gastric cancer
patients | 27 | 15 | 64.3 | 24.006 | <0.001 |
| Healthy
volunteers | 9 | 47 | 16.1 |
|
|
 | Table III.Association between TAP and
clinicopathological characteristics of gastric cancer patients. |
Table III.
Association between TAP and
clinicopathological characteristics of gastric cancer patients.
| Characteristics | Number | TAP-positive | TAP-negative | Positive rate
(%) | χ2 | P-value |
|---|
| Gender |
|
|
|
| 0.259 | 0.611 |
| Male | 30 | 20 | 10 | 66.7 |
|
|
|
Female | 12 | 7 | 5 | 58.3 |
|
|
| Age, years |
|
|
|
| 7.010 | 0.008 |
|
<60 | 12 | 4 | 8 | 33.3 |
|
|
| ≥60 | 30 | 23 | 7 | 76.7 |
|
|
| Localization |
|
|
|
| 0.196 | 0.907 |
|
Cardia | 21 | 13 | 8 | 61.9 |
|
|
| Body | 11 | 7 | 4 | 63.6 |
|
|
|
Antrum | 10 | 7 | 3 | 70.0 |
|
|
| Tumor size, cm |
|
|
|
| 0.058 | 0.810 |
|
<5 | 15 | 10 | 5 | 66.7 |
|
|
| ≥5 | 27 | 17 | 10 | 63.0 |
|
|
| Invasion depth |
|
|
|
| 0.494 | 0.482 |
|
T1-T3 | 8 | 6 | 2 | 75.0 |
|
|
| T4 | 34 | 21 | 13 | 61.8 |
|
|
|
Differentiation |
|
|
|
| 4.200 | 0.040 |
|
Well | 14 | 12 | 2 | 85.7 |
|
|
|
Poor | 28 | 15 | 13 | 53.6 |
|
|
| TNM stage |
|
|
|
| 6.741 | 0.034 |
|
I–II | 14 | 12 | 2 | 85.7 |
|
|
|
III | 7 | 2 | 5 | 28.6 |
|
|
| IV | 21 | 13 | 8 | 61.9 |
|
|
| LNM |
|
|
|
| 3.780 | 0.052 |
|
Positive | 32 | 18 | 14 | 56.3 |
|
|
|
Negative | 10 | 9 | 1 | 90.0 |
|
|
 | Table IV.Univariate analysis and multivariate
Cox regression analysis for PFS for all patients. |
Table IV.
Univariate analysis and multivariate
Cox regression analysis for PFS for all patients.
|
| Univariate
analysis | Multivariate Cox
regression analysis |
|---|
|
|
|
|
|---|
|
Characteristics | Median PFS
(months) | Log-rank
P-value | HR | 95% CI | P-value |
|---|
| All patients |
8.3 |
|
| Age, years |
|
|
<60 | 10.4 |
0.858 | 1.044 | 0.995–1.095 | 0.082 |
|
≥60 |
5.8 |
|
| Gender |
|
|
Male |
8.3 |
0.558 | 1.059 | 0.461–2.433 | 0.892 |
|
Female |
4.9 |
|
| TAP |
|
|
Positive |
4.2 |
0.043 | 64.487 | 11.905–349.315 | <0.001 |
|
Negative | 12.6 |
|
|
Differentiation |
|
|
Well | 12.6 |
0.009 | 17.279 | 4.504–66.296 | <0.001 |
|
Poor |
4.9 |
|
| TNM stage |
|
|
I–III | 14.0 | <0.001 | 45.480 | 9.370–220.758 | <0.001 |
| IV |
3.5 |
|
Discussion
As at present, the majority of patients with gastric
cancer succumb to the disease due to metastasis and recurrence,
early diagnosis and real-time monitoring of metastasis are
important to improve the survival time of the patients. Although
computed tomography (CT) and ultrasonography may be used to
identify metastasis in gastric cancer patients, they have
limitations and may not be sufficiently accurate (14,15). While
the predictive value of positron emission tomography-CT is high
with regard to local lymph node metastasis and distant metastasis
(16), this method is not affordable
for the majority of patients. Glycosylation has been indicated to
be an important factor at early stages of cancer development
(5). The present study demonstrated
that 12 of the 14 early-stage gastric cancer patients (85.7%) were
TAP-positive, supporting this role of TAP in the early stage of
gastric cancer. Furthermore, the present study indicated the
aptness of TAP detection for early diagnosis of gastric cancer and
may be applied for screening of high-risk populations in
combination with other diagnostic tools. He et al (17) revealed that TAP may be used as an
indicator for the diagnosis of lung cancer and for evaluating the
progress of lung cancer patients.
Serum tumor markers are characteristic substances
present in malignant cells or produced by abnormal malignant cells.
Several of the most frequently used tumor markers, including
carbohydrate antigen (CA)724, CA199, carcinoembryonic antigen (CEA)
and CA242, have been confirmed to jointly provide information
aiding in the diagnosis, classification, prognosis and treatment
selection in gastric cancer (18–21), while
the value of information provided by of any of them alone is low.
Jing et al (22) found that
the sensitivity of CA724, CA199, CEA and CA242 was only 25.4, 36.2,
26.8 and 42.9%, respectively. Therefore, it is desirable to
identify novel biomarkers with high sensitivity and specificity.
The TAP testing kit manufactured by Zhejiang Ruisheng Medical
Technology, Ltd. contains a combination of lectins, which can
recognize and bind to specific sugar molecules with high
specificity, thus interlinking a variety of abnormal glycoproteins
via their sugar chains to form characteristically shaped
crystalloid, which can then be observed using the TAP detection
image analyzer. The TAP detection system allows for combined
detection of several tumor markers in the same system, therefore
improving the sensitivity and specificity of detection. The overall
sensitivity and specificity of TAP detection in various types of
cancer patients are 85.8 and 80.2%, respectively (23). Jin et al (24) reported that the sensitivity and
specificity of TAP detection were 87.8 and 87.2%, respectively, for
patients with malignant tumors of the digestive system. In the
present study, 64.3% of gastric cancer patients were determined to
be TAP-positive, which was significantly higher than the percentage
of TAP-positive healthy volunteers (16.1%; P<0.01), indicating
that TAP has a higher sensitivity for the detection of gastric
cancer, which may be of significant diagnostic value. Numerous
factors have been confirmed to be associated with the prognosis of
gastric cancer patients, such as the pathological stage and the
tumor differentiation. Certain studies have shown a correlation
between changes in glycosylation and poor prognosis (25,26). The
present study showed that TAP, differentiation and TNM stage were
independent predictive factors for reduced PFS in gastric cancer
patients by multivariate Cox regression analysis (HR, 64.487,
P<0.01; HR, 17.279, P<0.01; HR: 45.480, P<0.01,
respectively). The median PFS of TAP-positive patients was
significantly correlated to a worse prognosis as compared to
TAP-negative patients (PFS, 4.2 vs. 12.6 months; P=0.043) according
to Kaplan-Meier survival curve analysis. Therefore, TAP was
indicated to be a risk factor in patients with gastric cancer.
Therapeutic interventions for TAP-positive patients are of great
practical significance for cancer prevention and treatment. Certain
anti-cancer vaccines based on glycans have produced promising
results (27,28).
The present study further showed that the
TAP-positive rate in gastric cancer patients aged ≥60 years (76.7%)
was significantly higher than that of patients aged <60 years
(33.3%; P=0.008). χ2 analysis showed that TAP was
positively correlated with patient age, which was consistent with
the findings of Shao et al (29), suggesting that screening of elderly
patients for TAP should be introduced in the clinical setting.
Moreover, significant differences in the TAP-positive rate were
identified between different TNM stages and grades of
differentiation (P=0.034 and 0.040, respectively). Of note, TAP
positivity was most prevalent among patients with
well-differentiated tumors and during the early stages of gastric
cancer; this finding may be attributed to the decreased metabolic
activity of the cancer cells during gastric cancer progression,
leading to decreased secretion of glycoproteins, so that TAP is no
longer detectable in advanced-stage patients. Finally, it was
revealed that the TAP status was not correlated with gender, tumor
location, tumor size, depth of invasion, or the presence of lymph
node metastasis (P>0.05).
It is worth mentioning that the present study had
certain limitations. The study cohort was relatively small and the
time to follow-up was short; therefore, the present study only
serves as a preliminary assessment of the correlation between TAP
and the PFS of gastric cancer patients. A further large-scale,
prospective, multicenter study is therefore required to confirm the
results of the present study. Wu et al (30) reported that TAP detection can be
utilized as a novel method for evaluating the efficacy of
chemotherapy in patients with metastatic colorectal cancer.
Similarly, Liu et al (31)
found that the TAP status was a factor for the prediction of the
efficacy of chemotherapy in gastric cancer patients with a higher
efficiency than that of conventional tumor markers. The TAP test
therefore holds promise in the monitoring of cancer patient
responses to chemotherapy, which requires further elucidation for
implementation in the clinic.
In conclusion, the present study indicated that the
TAP status is an independent predictive marker for PFS in patients
with gastric cancer. Furthermore, the TAP status was significantly
correlated with the tumor stage, patient age and tumor
differentiation. These findings, combined with those of previous
studies, indicated that TAP detection represents a promising
diagnostic and prognostic tool for gastric cancer and may also be
utilized for monitoring the response of patients to chemotherapy
response.
Acknowledgements
This study was partly supported by the Science and
Technology Planning Project of Changzhou, Jiangsu Province (no.
CE20135051), the Science and Technology Planning Project of
Changzhou Health Bureau (no. ZD201203 and ZD201301), the Research
Project of the Health Department of Jiangsu Province (no. Z201221),
the 333 Talents Training Project of Jiangsu Province, the Key
Medical Innovation Talents Training Project of Changzhou and the
Project of Jiangsu Province Sanitation Innovation Team (no.
LJ201157).
References
|
1.
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Allemani C, Weir HK, Carreira H, et al:
Global surveillance of cancer survival 1995–2009: Analysis of
individual data for 25,676,887 patients from 279 population-based
registries in 67 countries (CONCORD-2). Lancet. 385:977–1010. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Haverkamp L, Brenkman HJ, Seesing MF,
Gisbertz SS, van Berge Henegouwen MI, Luyer MD, Nieuwenhuijzen GA,
Wijnhoven BP, van Lanschot JJ, de Steur WO, et al: Laparoscopic
versus open gastrectomy for gastric cancer, a multicenter
prospectively randomized controlled trial (LOGICA-trial). BMC
Cancer. 15:5562015. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Yang S, Feng R, Pan ZC, Jiang T, Xu Q and
Chen Q: A comparison of intravenous plus intraperitoneal
chemotherapy with intravenous chemotherapy alone for the treatment
of gastric cancer: A meta-analysis. Sci Rep. 5:125382015.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hakomori S: Tumor malignancy defined by
aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer
Res. 56:5309–5318. 1996.PubMed/NCBI
|
|
6.
|
Hakomori S: Glycosylation defining cancer
malignancy: New wine in an old bottle. Proc Natl Acad Sci USA.
99:10231–10233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Moniaux N, Andrianifahanana M, Brand RE
and Batra SK: Multiple roles of mucins in pancreatic cancer, a
lethal and challenging malignancy. Br J Cancer. 91:1633–1638.
2004.PubMed/NCBI
|
|
8.
|
Dennis JW, Granovsky M and Warren CE:
Glycoprotein glycosylation and cancer progression. Biochim Biophys
Acta. 1473:21–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Li D, Mallory T and Satomura S: AFP-L3: A
new generation of tumor marker for hepatocellular carcinoma. Clin
Chim Acta. 313:15–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Meany DL and Chan DW: Aberrant
glycosylation associated with enzymes as cancer biomarkers. Clin
Proteomics. 8:72011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Debruyne EN, Vanderschaeghe D, Van
Vlierberghe H, Vanhecke A, Callewaert N and Delanghe JR: Diagnostic
value of the hemopexin N-glycan profile in hepatocellular carcinoma
patients. Clin Chem. 56:823–831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Meezan E, Wu HC, Black PH and Robbins PW:
Comparative studies on the carbohydrate-containing membrane
components of normal and virus-transformed mouse fibroblasts. II.
Separation of glycoproteins and glycopeptides by sephadex
chromatography. Biochemistry. 8:2518–2524. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Dube DH and Bertozzi CR: Glycans in cancer
and inflammation-potential for therapeutics and diagnostics. Nat
Rev Drug Discov. 4:477–488. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fairweather M, Jajoo K, Sainani N,
Bertagnolli MM and Wang J: Accuracy of EUS and CT imaging in
preoperative gastric cancer staging. J Surg Oncol. 111:1016–1020.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jee HB, Park MJ, Lee HS, Park MS, Kim MJ
and Chung YE: Is non-contrast CT adequate for the evaluation of
hepatic metastasis in patients who cannot receive iodinated
contrast media? PLoS One. 10:e01341332015. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Grabinska K, Pelak M, Wydmanski J,
Tukiendorf A and d'Amico A: Prognostic value and clinical
correlations of 18-fluorodeoxyglucose metabolism quantifiers in
gastric cancer. World J Gastroenterol. 21:5901–5909.
2015.PubMed/NCBI
|
|
17.
|
He P, Zhang M and Yuan F: The significance
of detecting tumor abnormal protein in patients with lung cancer. J
Hebei Med Univ. 10:1225–1226. 2012.
|
|
18.
|
Deng K, Yang L, Hu B, Wu H, Zhu H and Tang
C: The prognostic significance of pretreatment serum CEA levels in
gastric cancer: A meta-analysis including 14651 patients. PLoS One.
10:e01241512015. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zou L and Qian J: Decline of serum CA724
as a probable predictive factor for tumor response during
chemotherapy of advanced gastric carcinoma. Chin J Cancer Res.
26:404–409. 2014.PubMed/NCBI
|
|
20.
|
Zhu YB, Ge SH, Zhang LH, Wang XH, Xing XF,
Du H, Hu Y, Li YA, Jia YN, Lin Y, et al: Clinical value of serum
CEA, CA19-9, CA72-4 and CA242 in the diagnosis and prognosis of
gastric cancer. Chin J Gastrointest Surg. 15:161–164. 2012.(In
Chinese).
|
|
21.
|
Li F, Li S, Wei L, Liang X, Zhang H and
Liu J: The correlation between pre-operative serum tumor markers
and lymph node metastasis in gastric cancer patients undergoing
curative treatment. Biomarkers. 18:632–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Jing JX, Wang Y, Xu XQ, Sun T, Tian BG, Du
LL, Zhao XW and Han CZ: Tumor markers for diagnosis, monitoring of
recurrence and prognosis in patients with upper gastrointestinal
tract cancer. Asian Pac J Cancer Prev. 15:10267–10272. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ji LY: Tumor abnormal protein (TAP)
Application of screening for early cancer. China Health Care
Nutrition. 10:591–592. 2013.
|
|
24.
|
Jin H, Zhan HG and Cui X: Evaluation of
the effectiveness of tumor abnormal protein detection system in
digestive cancer. Mod Med J. 4:270–271. 2006.
|
|
25.
|
Miyake M, Taki T, Hitomi S and Hakomori S:
Correlation of expression of H/Le(y)/Le(b) antigens with survival
in patients with carcinoma of the lung. N Engl J Med. 327:14–18.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Nakamori S, Kameyama M, Imaoka S, Furukawa
H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y and
Irimura T: Increased expression of sialyl Lewisx antigen correlates
with poor survival in patients with colorectal carcinoma:
Clinicopathological and immunohistochemical study. Cancer Res.
53:3632–3637. 1993.PubMed/NCBI
|
|
27.
|
Krug LM, Ragupathi G, Ng KK, Hood C,
Jennings HJ, Guo Z, Kris MG, Miller V, Pizzo B, Tyson L, et al:
Vaccination of small cell lung cancer patients with polysialic acid
or N-propionylated polysialic acid conjugated to keyhole limpet
hemocyanin. Clin Cancer Res. 10:916–923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Holmberg LA and Sandmaier BM: Vaccination
with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev
Vaccines. 3:655–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Shao JY and Shi NF: Analysis of early
malignant tumor using tumor abnormal protein screening. Dis
Surveill. 09:6452007.
|
|
30.
|
Wu XY and Huang XE: Clinical application
of serum tumor abnormal protein (TAP) in colorectal cancer
patients. Asian Pac J Cancer Prev. 16:3425–3428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Liu J and Huang XE: Clinical application
of serum tumor abnormal protein from patients with gastric cancer.
Asian Pac J Cancer Prev. 16:4041–4044. 2015. View Article : Google Scholar : PubMed/NCBI
|