Introduction
Glioblastoma (GB), a frequent type of malignant
glioma, is the most common primary brain tumor and is associated
with a poor prognosis. Despite aggressive multimodality treatments,
including cytoreductive surgery, radiotherapy (RT) and systemic
chemotherapy, GB recurs and it is invariably fatal. Additional
therapeutic strategies are urgently required to elicit prolong
tumor control and patient survival (1).
A carmustine (bis-chloroethylnitrosourea, BCNU; an
alkylating agent of the nitrosourea family) wafer
(Gliadel®; Eisai Inc., Tokyo, Japan) is a
controlled-release preparation of BCNU that is implanted into the
brain (2). Carmustine wafers, lined
along the wall of the resection cavity following tumor removal,
exert antitumor effects on the residual tumor as adjuvant local
chemotherapy. This implant has been shown to enhance the overall
survival of patients with malignant gliomas in controlled clinical
trials in the United States and Europe (3). A prospective, multicenter phase-I/II
study on Japanese patients was recently performed and the
application of carmustine wafers has been covered by Japanese
public health insurance since 2012 (2).
Through local application, an increased
concentration of carmustine may be delivered to the tumor bed over
a period of ≥3 weeks, during which, local reactions caused by this
chemotherapeutic may occur (4).
Previous studies have described distinctive changes on computed
tomography (CT) and magnetic resonance (MR) imaging, including
brain edema, gas retention and cyst formation, almost all of which
are frequently discussed as adverse events and complications
(4–7).
To the best of our knowledge, favorable responses during the acute
phase have not been reported to date.
We herein present the first reported case of GB,
which rapidly regressed, as observed on CT and MRI scans, following
partial tumor removal and carmustine wafer implantation. Based on
the example of this case, the pharmacological prediction and
surgical indication of carmustine wafer therapy were also
discussed.
Case report
History
A 77-year-old Japanese woman presented with speech
disturbance that aggravated over the next month, followed by gait
unsteadiness. The patient had a past history of chronic obstructive
pulmonary disease, which required medical treatment at that
time.
Examination
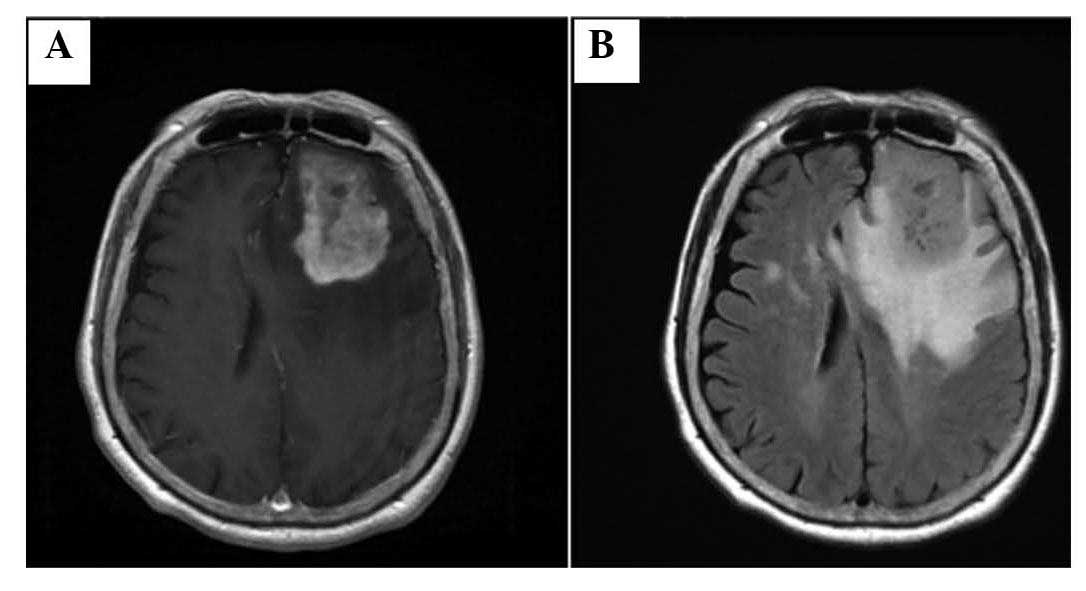
The physical examination revealed mild motor aphasia
and right hemiparesis. MRI revealed a tumor in the left frontal
lobe of the brain (Fig. 1).
Operation
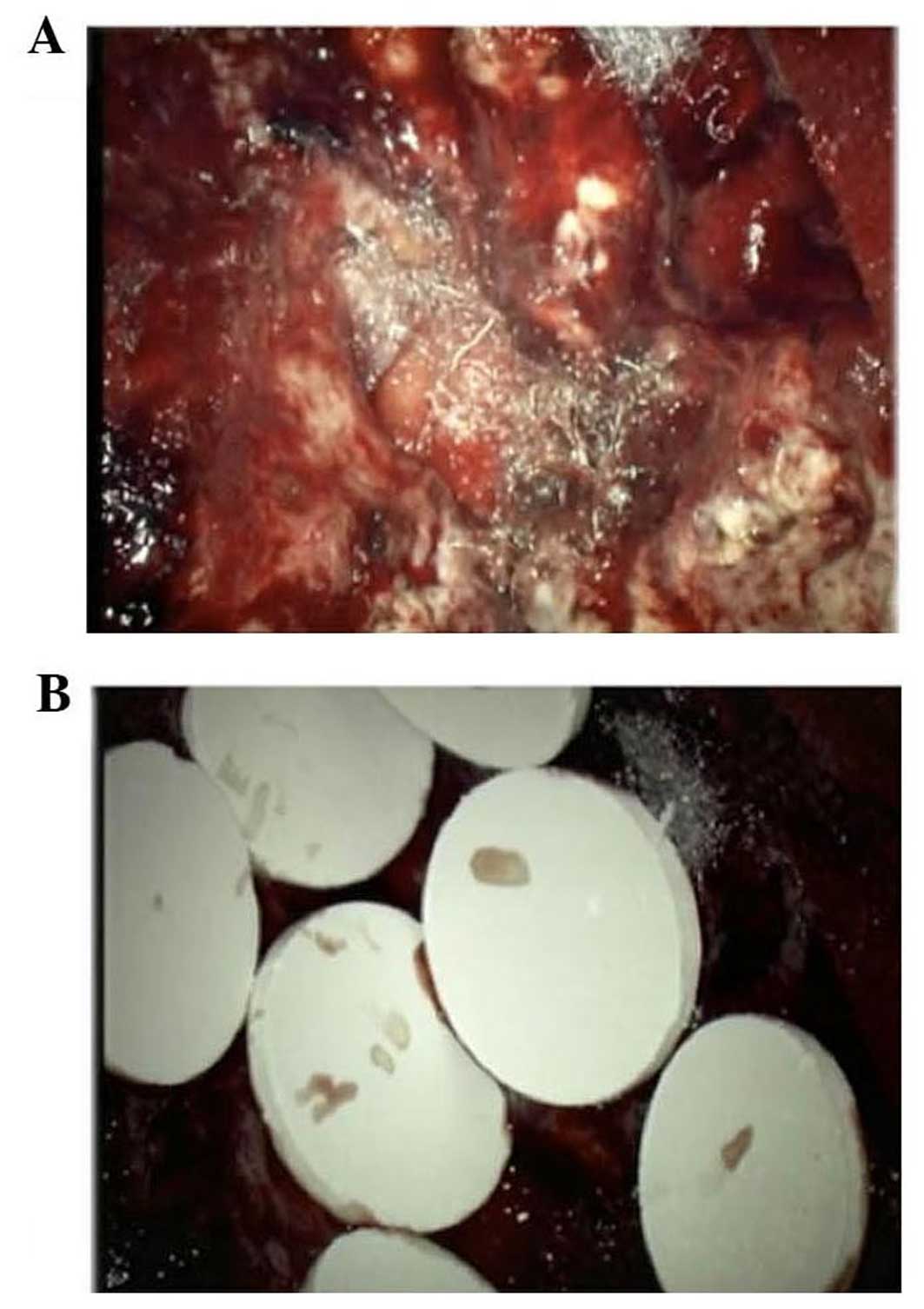
Due to its eloquent location, the tumor was
partially removed under 5-aminolevulinic acid fluorescence guidance
and 8 carmustine wafers were implanted in the resection cavity
(Fig. 2).
Pathological findings
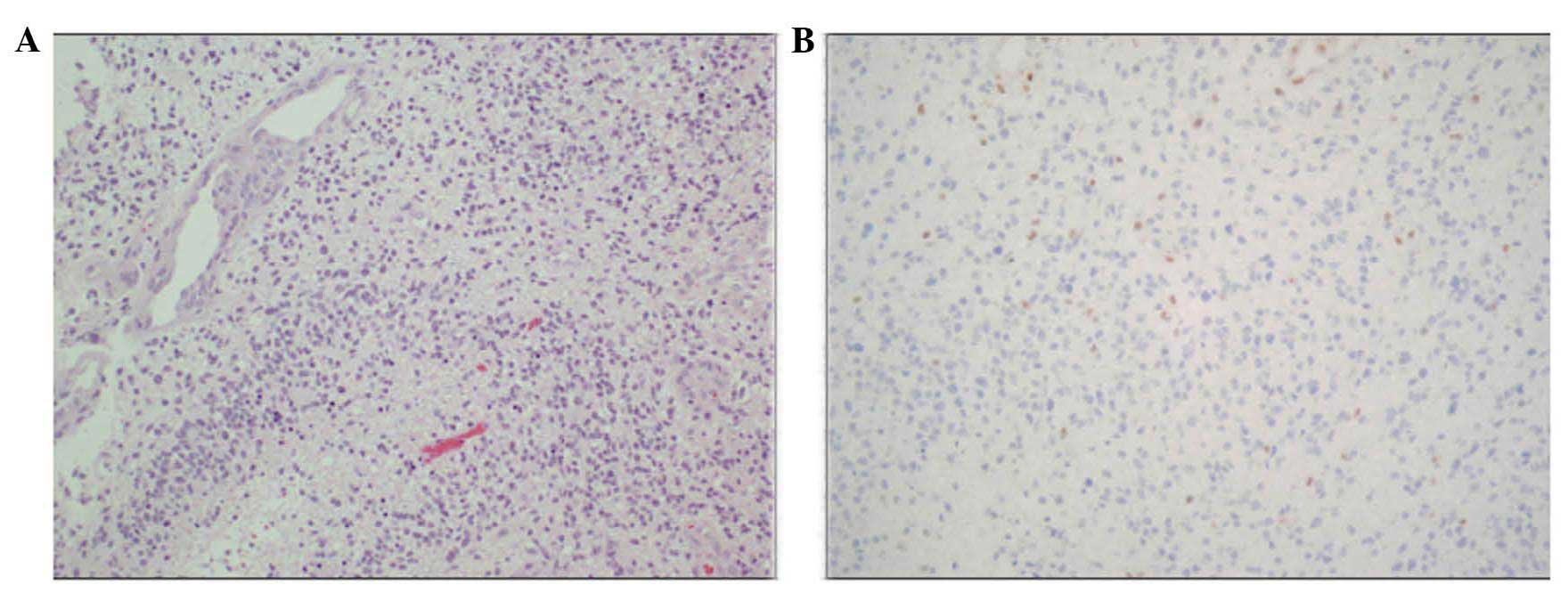
Histological examination revealed a highly cellular
tumor composed of atypical glial cells (Fig. 3A). Mitoses, necrosis and microvascular
proliferation were observed. The tumor cells were scarcely
immunoreactive for O6-methylguanine-DNA
methyltransferase (MGMT) (Fig. 3B).
The molecular immunology Borstel-1 (MIB-1) staining index was ~10%.
Genetic analysis revealed a methylated MGMT gene promoter
and wild-type isocitrate dehydrogenase (IDH)1 and −2 genes
(Table I). These pathological
findings suggested the diagnosis of GB.
 | Table I.Genetic analysis of the present
case. |
Table I.
Genetic analysis of the present
case.
| Genetic
alteration | Status |
|---|
| MGMT promoter
methylation | (+) |
| IDH1/2
mutation | (−) |
| P53
mutation | (−) |
| H3F3A
mutation | (−) |
| HIST1H3B
mutation | (−) |
Postoperative course
Postoperative CT and MRI scans revealed the presence
of residual disease and perifocal edema with a mass effect
(Fig. 4). Postoperatively, steroids
were administered and no additional deterioration was observed.
Adjuvant treatment was planned: 150–200 mg/m2/day
temozolomide (TMZ) for 5 days every 4 weeks without radiation until
disease progression. Shortly after the initiation of TMZ
chemotherapy, adverse effects, including fever and diarrhea,
developed and TMZ was discontinued. At that time, only 300 mg of
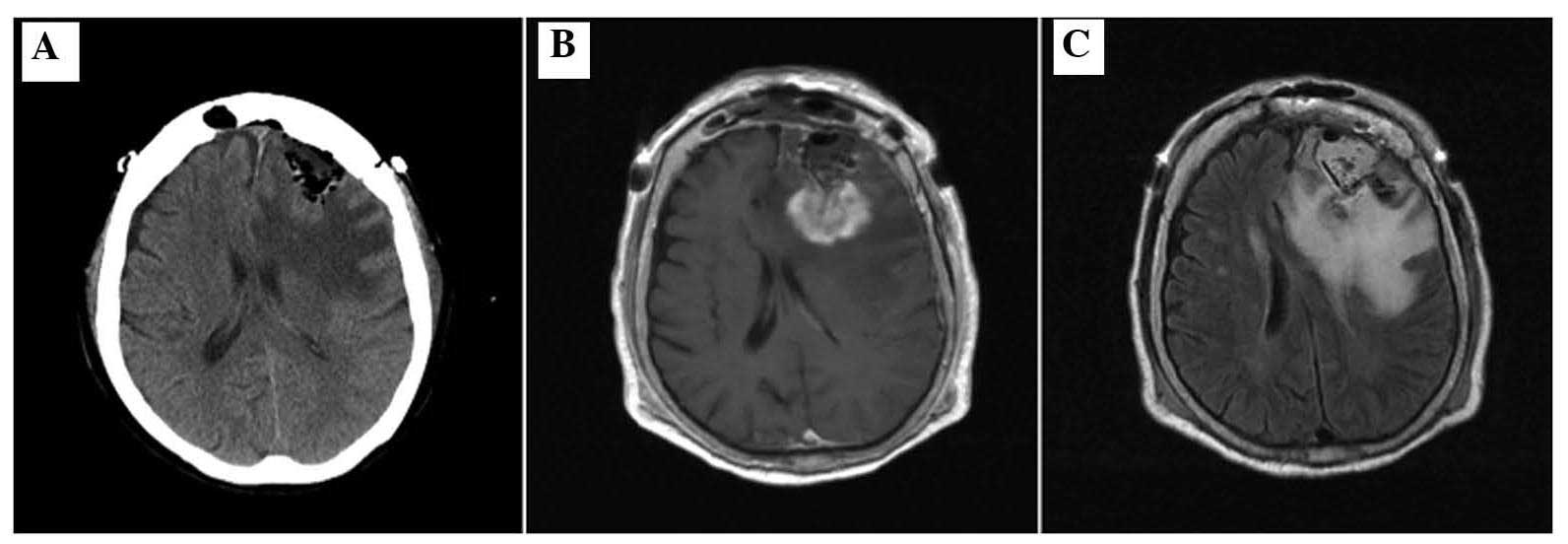
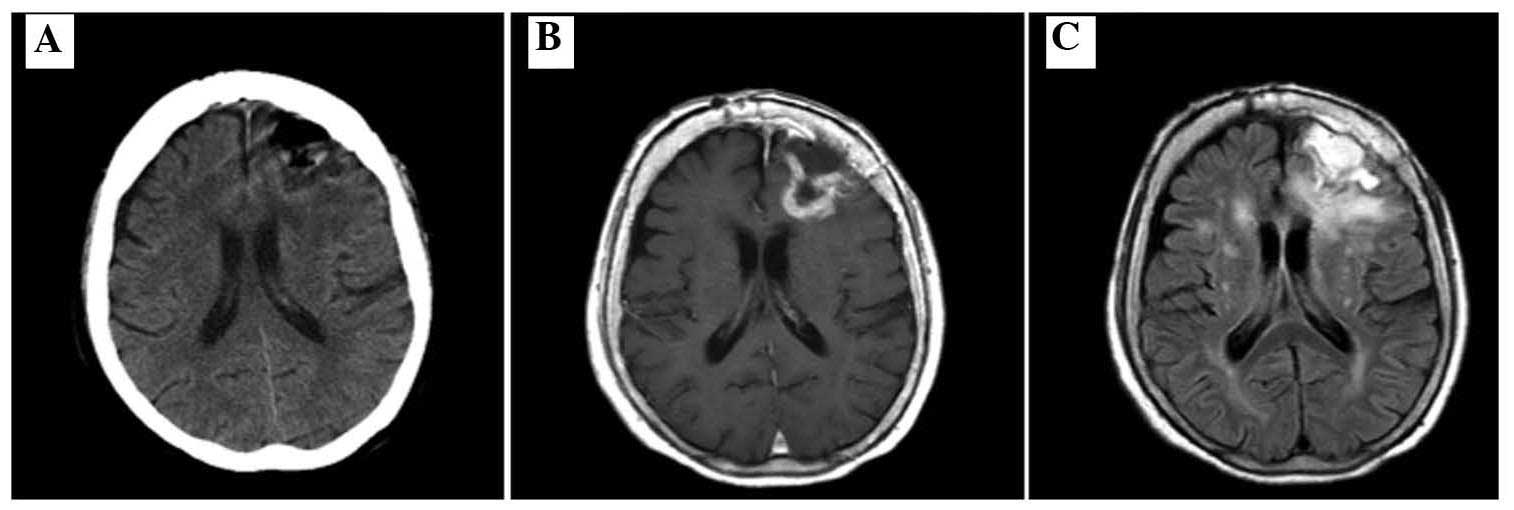
TMZ had been administered in total. A CT scan after 1 week revealed
a decreased mass effect of the lesion (Fig. 5A) and a subsequent MRI revealed marked
regression of the residual tumor and perifocal edema (Fig. 5B and C). After the patient's general
condition improved (~3.5 months after operation), adjuvant TMZ was
re-introduced every 2 months, until disease progression. The
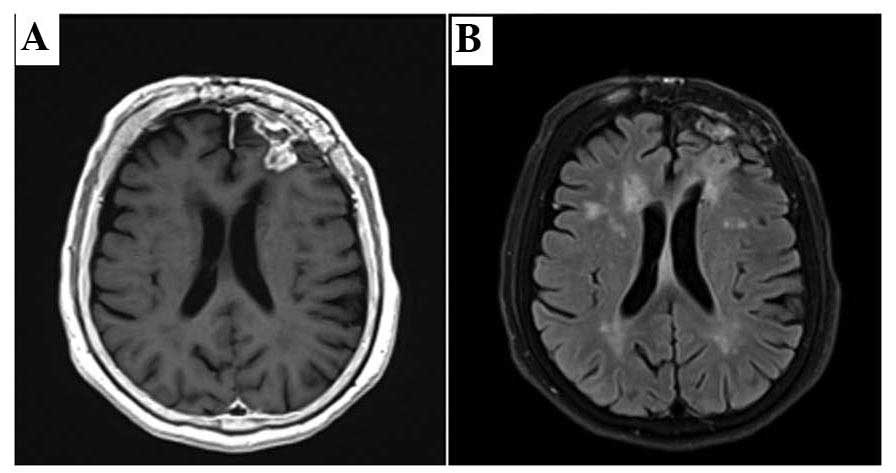
treatment was beneficial, the tumor regressed and the patient's
symptoms and signs resolved (Fig. 6).
The patient remains progression-free for >12 months after the
operation.
Ethical approval and informed
consent
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images. The protocol of the study was ethically approved by the
Institutional Review Board of the Wakayama Medical University
(permit no. 61).
Discussion
For low- as well as high-grade gliomas, extensive
surgical resection is associated with longer patient survival
(8). However, the extent of resection
in eloquent areas of the brain is limited, in order to avoid the
development of additional neurological deficits and performance
deterioration. The residual tumor should be controlled by
additional treatment modalities, including radiation and/or
chemotherapy. Carmustine wafer implantation in the resection cavity
containing the residual tumor may be promising as local
chemotherapy. The present case demonstrated an unexpected rapid
regression and good local control, despite only partial removal of
the tumor. We hypothesize that this favourable result within only 1
month postoperatively is mainly attributed to the carmustine
wafers.
RT plus concomitant and adjuvant TMZ, an alkylating
agent, currently represents the standard of care for newly
diagnosed GB patients (9).
Consequently, carmustine wafers may be implanted concomitantly with
these combined standard treatments. The rationale underlying this
type of local therapy is to fill the gap between surgery and
adjuvant treatments to maintain alkylating treatment pressure on
residual tumor cells (10). However,
in the present case, carmustine wafers were so effective that the
residual tumor had markedly regressed when systemic TMZ
chemotherapy was initiated, indicating that the outcome of adjuvant
therapy was mainly affected by the carmustine wafers.
Methylation of the MGMT gene promoter and low
expression of the MGMT protein have been associated with
responsiveness to systemic carmustine chemotherapy, an increase in
overall survival and the time-to-progression of the disease
(11). A pharmacokinetic analysis
indicated that exposure of the tissue area to carmustine under a
concentration-time curve achieved by polymeric delivery was
4–1,200-fold higher compared with that produced by intravenous
administration of a higher dose; however, the MGMT status should be
considered as the main factor that limits the efficacy of
carmustine wafers (12). Indeed, in
patients with newly diagnosed GB who underwent surgical resection
and received carmustine wafer implants followed by adjuvant
radiotherapy and concomitant oral TMZ chemotherapy, MGMT
promoter methylation status and low MGMT expression were identified
as positive prognostic factors (10).
In the present case, the tumor was highly responsive to carmustine
wafers, and also exhibited genetic characteristics conferring
sensitivity to alkylating agents.
Previous pharmacokinetic studies on animals and
associated modelling demonstrated the ability of carmustine wafers
to produce high-dose delivery (millimolar concentrations) within
millimeters of the polymer implant, with a limited penetration
distance of carmustine from the site of delivery (13). In addition, the presence of
significant doses of carmustine in more distant regions of the
brain (centimeters away from the carmustine wafer implant) has been
shown to persist over the course of 1 week in non-human primates
(13). However, Fung et al
(12) revealed that a significant
concentration of carmustine (0.4 µM) was detected at up to 5 cm
from the implant as late as 30 days after implantation, although a
high drug concentration (0.5–3.5 mM) was measured within 3 mm.
Therefore, it may be preferable for carmustine wafers to be placed
as close to the residual tumor as possible. We consider that
effective placement of wafers was accomplished in the present
case.
For GB patients subjected to ≥90% resection in the
BCNU wafer study, the median survival increased compared with that
of placebo-treated controls, while no further survival increase was
found for cases with <90% resection (14). However, to date, no evidence-based
recommendations are available to guide implantation decisions based
on the extent of tumor resection (15). Gutenberg et al (15) recommended that wafer implantation
should only be planned if gross total resection appears feasible,
if tumor resection leads to a significant reduction of the mass
effect and if the resulting resection cavity is of sufficient size
for wafer implantation. As demonstrated in the present case,
although the tumor was only partially resected, it was considered
that carmustine wafers could be implanted if the mass effect was
significantly reduced and the resection cavity was of sufficient
size. However, one should always consider the possibility of brain
edema, resulting in severe, albeit transient, neurological deficits
(15).
Although surgical resection followed by RT with
concurrent and adjuvant TMZ is the standard of care for non-elderly
patients with GB, the safety and efficacy of these modalities in
elderly patients are less certain, as this population is
underrepresented in several clinical trials (16). According to the recent literature,
however, the use of TMZ in elderly patients appears to be safe,
when administered to, or as a substitute for, RT (16). In particular, among elderly patients
with GB harboring MGMT promoter methylation, the addition of
adjuvant TMZ to RT or the substitution of TMZ for RT is likely to
prolong survival compared with RT alone (16–18). Due
to the marked toxicity of available therapies and increased
prevalence of comorbidities in elderly patients, the ability of
each individual patient to receive treatment requires consideration
prior to treatment. In the present case, adjuvant TMZ monotherapy
was reasonable and beneficial, considering the performance status
and genetic profile of the patient.
In conclusion, we herein reported the case of an
elderly patient with GB in an eloquent region of the brain (left
frontal lobe). Despite the opinion that a palliative treatment
strategy may be more appropriate compared with an aggressive
multimodal regimen in such a case, partial removal of the tumor was
followed by placement of carmustine wafers (15). As a result, marked regression of the
residual tumor was observed, without any adverse effects, within a
short time after surgery. The present case demonstrated a marked
antitumor effect of carmustine wafers, which, to the best of our
knowledge, had not been reported to date. Considering the possible
adverse events, carmustine wafer implantation may be planned if the
size of the resulting resection cavity is sufficient for wafer
placement, even if total gross resection does not appear to be
feasible.
Glossary
Abbreviations
Abbreviations:
|
MGMT
|
O6-methylguanine-DNA
methyltransferase
|
|
GB
|
glioblastoma
|
|
RT
|
radiotherapy
|
|
BCNU
|
bis-chloroethylnitrosourea
|
|
MG
|
malignant gliomas
|
|
MR
|
magnetic resonance
|
|
IDH
|
isocitrate dehydrogenase
|
|
TMZ
|
temozolomide
|
References
|
1
|
Broniscer A, Tatevossian RG, Sabin ND, et
al: Clinical, radiological, histological, and molecular
characteristics of paediatric epithelioid glioblastoma. Neuropathol
Appl Neurobiol. 40:327–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aoki T, Nishikawa R, Sugiyama K, Nonoguchi
N, Kawabata N, Mishima K, Adachi J, Kurisu K, Yamasaki F, Tominaga
T, et al: A multicenter phase I/II study of the BCNU implant
(Gliadel® Wafer) for Japanese patients with malignant
gliomas. Neurol Med Chir (Tokyo). 54:290–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Westphal M, Hilt DC, Bortey E, Delavault
P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J and Ram Z: A
phase 3 trial of local chemotherapy with biodegradable carmustine
(BCNU) wafers (Gliadel wafers) in patients with primary malignant
glioma. Neurol Oncol. 5:79–88. 2003. View Article : Google Scholar
|
|
4
|
Ulmer S, Spalek K, Nabavi A, Schultka S,
Mehdorn HM, Kesari S and Dörner L: Temporal changes in magnetic
resonance imaging characteristics of Gliadel wafers and of the
adjacent brain parenchyma. Neuro Oncol. 14:482–490. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colen RR, Zinn PO, Hazany S, Do-Dai D, Wu
JK, Yao K and Zhu JJ: Magnetic resonance imaging appearance and
changes on intracavitary Gliadel wafer placement: A pilot study.
World J Radiol. 3:266–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hammoud DA, Belden CJ, Ho AC, Dal Pan GJ,
Herskovits EH, Hilt DC, Brem H and Pomper MG: The surgical bed
after BCNU polymer wafer placement for recurrent glioma: Serial
assessment on CT and MR imaging. AJR Am J Roentgenol.
180:1469–1475. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prager JM, Grenier Y, Cozzens JW,
Chiowanich P, Gorey MT and Meyer JR: Serial CT and MR imaging of
carmustine wafers. AJNR Am J Neuroradiol. 21:119–123.
2000.PubMed/NCBI
|
|
8
|
Sanai N and Berger MS: Glioma extent of
resection and its impact on patient outcome. Neurosurgery.
62:753–764; discussion 264–266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukai J, Koizumi F and Nakao N: Enhanced
anti-tumor effect of zoledronic acid combined with temozolomide
against human malignant glioma cell expressing O6-methylguanine DNA
methyltransferase. PLoS One. 9:e1045382014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lechapt-Zalcman E, Levallet G, Dugué AE,
et al: O(6)-methylguanine-DNA methyltransferase (MGMT) promoter
methylation and low MGMT-encoded protein expression as prognostic
markers in glioblastoma patients treated with biodegradable
carmustine wafer implants after initial surgery followed by
radiotherapy with concomitant and adjuvant temozolomide. Cancer.
118:4545–4554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esteller M, Garcia-Foncillas J, Andion E,
et al: Inactivation of the DNA-repair gene MGMT and the clinical
response of gliomas to alkylating agents. N Engl J Med.
343:1350–1354. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fung LK, Ewend MG, Sills A, Sipos EP,
Thompson R, Watts M, Colvin OM, Brem H and Saltzman WM:
Pharmacokinetics of interstitial delivery of carmustine,
4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable
polymer implant in the monkey brain. Cancer Res. 58:672–684.
1998.PubMed/NCBI
|
|
13
|
Fleming AB and Saltzman WM:
Pharmacokinetics of the carmustine implant. Clin Pharmacokinet.
41:403–419. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stummer W, van den Bent MJ and Westphal M:
Cytoreductive surgery of glioblastoma as the key to successful
adjuvant therapies: New arguments in an old discussion. Acta
Neurochir (Vienna). 153:1211–1218. 2011. View Article : Google Scholar
|
|
15
|
Gutenberg A, Lumenta CB, Braunsdorf WE,
Sabel M, Mehdorn HM, Westphal M and Giese A: The combination of
carmustine wafers and temozolomide for the treatment of malignant
gliomas. A comprehensive review of the rationale and clinical
experience. J Neurooncol. 113:163–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jordan JT, Gerstner ER, Batchelor TT,
Cahill DP and Plotkin SR: Glioblastoma care in the elderly. Cancer.
122:189–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malmström A, Grønberg BH, Marosi C, et al:
Temozolomide versus standard 6-week radiotherapy versus
hypofractionated radiotherapy in patients older than 60 years with
glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol.
13:916–926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wick W, Platten M, Meisner C, et al:
Temozolomide chemotherapy alone versus radiotherapy alone for
malignant astrocytoma in the elderly: The NOA-08 randomised, phase
3 trial. Lancet Oncol. 13:707–715. 2012. View Article : Google Scholar : PubMed/NCBI
|