Introduction
Sunitinib is a tyrosine kinase inhibitor
(Sutent® Capsules; Pfizer, Kent, UK), approved by the
European Medicines Agency for the treatment of unresectable or
metastatic gastrointestinal stromal tumors and renal cell
carcinoma.
Sunitinib inhibits several tyrosine kinases,
including vascular endothelial growth factor receptor (VEGFR) and
platelet-derived growth factor receptor (PDGFR). Simultaneous
inhibition of PDGFR and VEGFR has a crucial role in reducing tumor
vascularization and tumor cell proliferation (1). The most common adverse events associated
with sunitinib are asthenia and gastrointestinal toxicity. Specific
adverse drug reactions include skin discoloration, as well as
bleeding and proteinuria, due to the inhibition of angiogenesis
(2). Inhibition of VEGF impairs
tissue repair and renewal of endothelial cells, and may lead to an
elevated risk of bleeding. The overall incidence of hemorrhage (all
grades) is 19.3%. However, severe hemorrhage is rare, with an
incidence of 3% for grade 3–5 events (3).
Additionally, sunitinib can induce myelosuppression
(4). According to the summary of
product characteristics, thrombocytopenia occurs in 8.9% of
patients.
The present study described a case of disseminated
intravascular coagulation, associated with the administration of
sunitinib. To the best of our knowledge, this is the first report
of this adverse event.
Case report
A 34-year-old male patient with Type I diabetes was
undergoing treatment for invasive thymoma (diagnosed in 2012) with
pulmonary, bone, liver and pleural metastases. For >1 year, the
patient had been suffering from fever, a chronic inflammatory
syndrome and anemia, requiring regular red blood cell
transfusions.
The patient initially received etoposide-cisplatin,
followed by cisplatin-doxorubicin-cyclophosphamide. This treatment
produced a partial response, however, the patient developed chronic
kidney disease as a result of cisplatin administration and
diabetes. The disease recurred in February 2014. A further course
of etoposide-carboplatin was initiated, however, did not prevent
disease progression within the liver. Biopsy of a liver nodule
revealed metastases of thymic carcinoma. Treatment with sunitinib
was initiated in July 2014, termed as day 0. A low dose (25
mg/daily) was used since the patient had an Eastern Cooperative
Oncology Group performance status of 3. The patient also received
amlodipine, esomeprazole, insulin and furosemide. Upon initiation
of sunitinib, the results of the complete blood count and
coagulation tests were normal: The blood levels of hemoglobin (9.2
g/dl), creatinine (189 µmol/l) and fibrinogen (8 g/l). Two weeks
later, the daily dose of sunitinib was increased to 37.5 mg. A
computed tomography scan on day 21 showed that the disease was
stable. The patient soon complained of edema of the lower limbs and
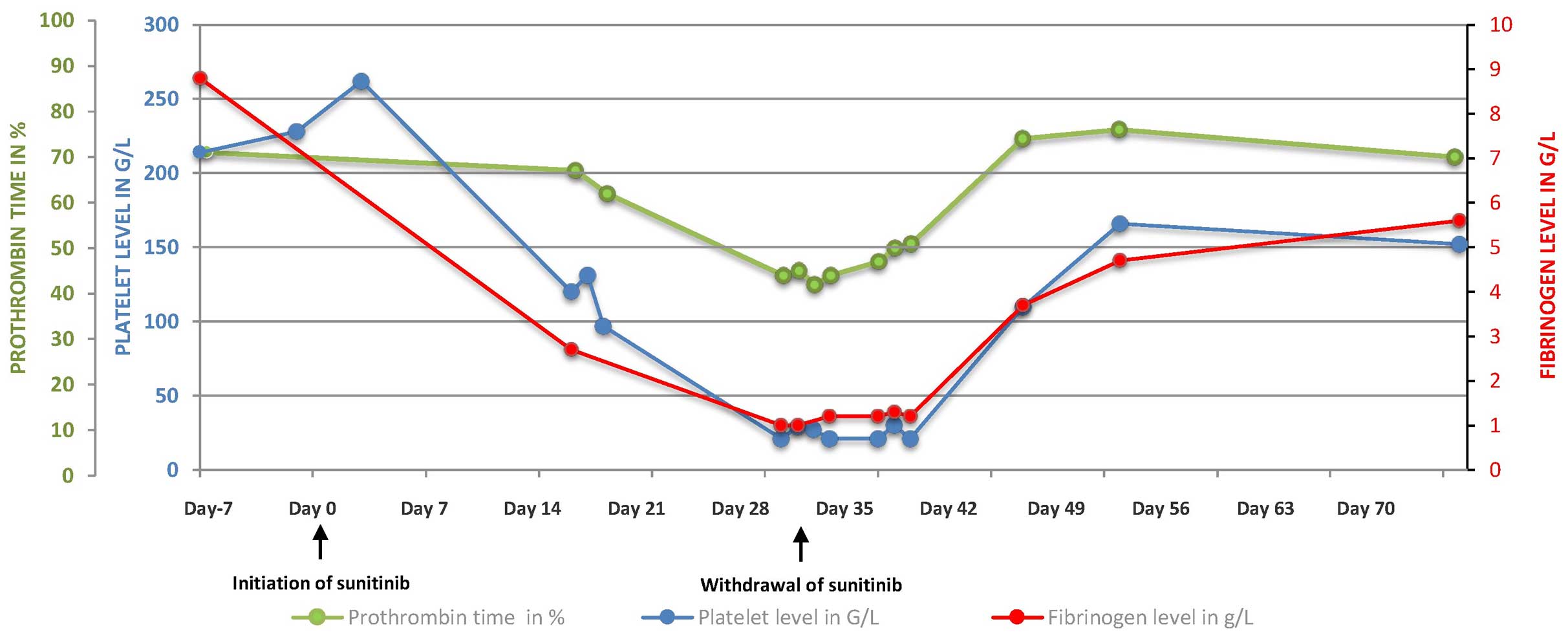
the platelet count fell to 100 G/l after three weeks of treatment
(Fig. 1).
On day 28, the patient was admitted to the Emergency
Department after noticing spots of blood on his underwear. No
evidence of gastrointestinal or urinary hemorrhage was observed.
The body temperature and blood pressure were normal. The patient
complained about worsening of the white, soft, painless edema of
the lower limbs over the previous month. Neither proteinuria nor
hypertension was observed, thus ruling out nephrotic syndrome. A
hematological assessment on day 29 revealed bicytopenia, with a
hemoglobin level of 6.1 g/dl, a platelet count of 21 G/l and a
white blood cell count of 4.9 G/l (Fig.
1). The levels of haptoglobin and unconjugated bilirubin were
normal, thus ruling out hemolysis. No schistocytosis was diagnosed.
The present study diagnosed disseminated intravascular coagulation
(DIC), on the basis of thrombocytopenia, a low prothrombin time
(PT; 45%) and low levels of factors II (65%), V (44%), VII (57%)
and X (121%), and fibrinogen (1.3 g/l). The patient received four
red blood cell units and two platelet units. Sunitinib was
withdrawn. The hematological situation worsened, with an increased
requirement for transfusion and the development of purpuric lesions
on the left arm, purpuric and ecchymotic lesions in the abdomen,
and bruising at the injection site. The levels of coagulation
factors fell to <50%, with the exception of factor II, at 57%),
with soluble complexes >150 µg/ml and fibrin degradation
products >40 µg/ml. The nadirs of the platelet count and the
hemoglobin level were 21 G/l and 5.7 g/dl, respectively.
The DIC observed may potentially have been
associated with tumor progression, with possible bone marrow
involvement, infectious disease or an iatrogenic effect of
sunitinib. A bone marrow aspirate revealed a moderately rich marrow
and a normal megakaryocyte profile, with no malignant cells and no
hemophagocytosis. Bacterial cultures were negative. The patient
received a further four red blood cell units and two units of
platelet concentrate. The withdrawal of sunitinib and the
initiation of hematological support were followed by a slow
improvement in the clinical signs of hemorrhagic syndrome, which
resolved 10 days later. The platelet counts, PT and fibrinogen
levels normalized within 15 days. Once the DIC had resolved, a
sixth course of treatment with paclitaxel was initiated 6 weeks
after the final administration of sunitinib. Although no recurrence
of DIC was reported, the patient became neutropenic and succumbed
to septic shock. The present study, therefore, suspected sunitinib
to be causally associated with the severe hematological toxicity
associated with DIC.
Discussion
To the best of our knowledge, this is the first
report of DIC with a probable causal association with
sunitinib.
The initial toxic effects of sunitinib (lower limb
edema) appeared early in the course of treatment and worsened
following an increase in the dose. The edema may have been due to
the inhibition of PDGF, which is known to be involved in fluid
homeostasis (5), or to
hypoalbuminemia. Disseminated intravascular coagulation developed
within 4 weeks of therapy. The usual causes of DIC, bacterial
sepsis and bone marrow invasion by malignant cells, were absent
(6). Sunitinib was the only drug
prescribed after July 2014. Therefore, after eliminating other
likely causes, it was hypothesized that sunitinib had an iatrogenic
effect, as evidenced by the reduction in clinical and biological
symptoms, including complete normalization of coagulation
parameters in the context of metastatic carcinoma, following
treatment discontinuation. Therefore, regular monitoring of the
complete blood count and coagulation parameters is advisable in
patients treated with sunitinib, according to the summary of
product characteristics (7).
References
|
1
|
Aparicio-Gallego G, Blanco M, Figueroa A,
García-Campelo R, Valladares-Ayerbes M, Grande-Pulido E and
Antón-Aparicio L: New insights into molecular mechanisms of
sunitinib-associated side effects. Mol Cancer Ther. 10:2215–2223.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kollmannsberger C, Soulieres D, Wong R,
Scalera A, Gaspo R and Bjarnason G: Sunitinib therapy for
metastatic renal cell carcinoma: recommendations for management of
side effects. Can Urol Assoc J. 1:S41–S54. 2007.PubMed/NCBI
|
|
3
|
Je Y, Schutz FA and Choueiri TK: Risk of
bleeding with vascular endothelial growth factor receptor
tyrosine-kinase inhibitors sunitinib and sorafenib: A systematic
review and meta-analysis of clinical trials. Lancet Oncol.
10:967–974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar R, Crouthamel MC, Rominger DH,
Gontarek RR, Tummino PJ, Levin RA and King AG: Myelosuppression and
kinase selectivity of multikinase angiogenesis inhibitors. Br J
Cancer. 101:1717–1723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giles FJ, O'Dwyer M and Swords R: Class
effects of tyrosine kinase inhibitors in the treatment of chronic
myeloid leukemia. Leukemia. 23:1698–1707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levy M: Disseminated intravascular
coagulation: What's New? Crit Care Clin. 21:449–467. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valle JW, Faivre S, Hubner RA, Grande E
and Raymond E: Practical management of sunitinib toxicities in the
treatment of pancreatic neuroendocrine tumors. Cancer Treat Rev.
40:1230–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|