Introduction
Endoscopy is the gold standard for diagnosing
cancers of the gastrointestinal tract, including the esophagus,
stomach, colon and rectum (1,2). However, endoscopy is not suitable for
evaluating the depth of invasion and extent of cancer, as it only
allows observation of the lumen. Endoscopic ultrasonography (EUS)
and contrast-enhanced computed tomography (CE-CT) are performed to
assess the structure of the primary lesion, the depth of invasion
into the surrounding tissues and distant metastasis (3,4). Assessing
the depth of invasion may occasionally be difficult due to the weak
contrast of the cancer against the surrounding tissues. Therefore,
an imaging modality with a strong signal and contrast would
facilitate the assessment of the depth of tumor invasion.
Magnetic resonance imaging (MRI) is not as popular
as CT due to blurring and low spatial resolution (5). However, MRI may be a promising method if
a strong soft tissue contrast in the abdomen can be achieved.
Diffusion-weighted whole-body imaging with background body signal
suppression (DWIBS) images are acquired using multiple-signal
averaging, pre-pulse fat suppression and heavy diffusion weighting
during free breathing (6). DWIBS is
based on diffusion-weighted imaging (DWI) that visualizes and
assesses the random movement of water at the molecular level
(Brownian motion) (7,8). An advantage of DWIBS is that it provides
a strong contrast of cancerous against surrounding non-cancerous
tissues, which is useful for the detection, staging and monitoring
of the response to therapy (9). A
major limitation of DWIBS is that anatomical analysis may be
difficult at times (10,11). Fusion images of DWIBS and T2-weighted
images (T2WI (DWIBS/T2) are created by overlapping DWIBS with T2WI
using a workstation (9,12,13).
DWIBS/T2 therefore clearly illustrates functional information in
anatomical images.
In the present study, the performance of DWIBS/T2 in
the diagnosis of gastrointestinal cancers was retrospectively
analyzed.
Patients and methods
Ethical statement
The present study was approved by the Ethics
Committee of the National Hospital Organization Shimoshizu Hospital
(Yotsukaido, Japan). This was not considered a clinical trial, as
the procedures were performed as a part of routine clinical
practice. Written informed consent was obtained from all patients
who were subjected to MRI, upper gastrointestinal endoscopy,
colonoscopy and CE-CT. Consent was obtained from patients who were
subjected to abdominal ultrasonography, but written form was
waived. Written informed consent for inclusion into the study was
also waived, as patient records were anonymized and retrospectively
analyzed.
Study design
Patient records, including imaging, from July, 2012
until June, 2013 were retrospectively analyzed. The patients were
subjected to upper gastrointestinal endoscopy to investigate
abdominal pain, anemia, hematemesis and other symptoms suggesting
diseases of the esophagus, stomach or duodenum. The patients were
subjected to colonoscopy for the investigation of abdominal pain,
melena and other symptoms suggesting diseases of the colon or
rectum. A proportion of the patients had been subjected to upper
gastrointestinal endoscopy and colonoscopy as part of screening.
The patient inclusion criteria were as follows: i) Pathological
diagnosis of esophageal, gastric or colon cancer based on bioptic
or endoscopic mucosal resection specimens; ii) available DWIBS/T2
images. A total of 8 men (mean age, 71.6±12.5 years; range 67–77)
and 8 women (mean age, 71.6±4.0 years; range, 46–82) were enrolled
in the present study. The depth of invasion and tumor diameter were
assessed based on specimens obtained through surgery or endoscopic
mucosal resection. T staging was performed using CE-CT, abdominal
ultrasonography or EUS, according to the 7th edition of the
American Joint Committee on Cancer classification (14).
MRI
All MRI studies were performed using a 1.5 Tesla
scanner (Achieva, software version 3.2.2, Philips Medical Systems,
Best, The Netherlands). T1-weighted image (T1WI), T2WI and DWI were
obtained with pulse sequences, as depicted in Table I. DWIBS/T2 images were constructed
with Extended MR WorkSpace (Philips, Best, The Netherlands). The
DWI gradients were applied along the X, Y and Z axes before and
after a 180° inversion pre-pulse to obtain fat-saturated, isotropic
images with DWI sensitivity using the following parameters for a
single stack: b-value, 0 mm2/sec and 800
mm2/sec; repetition time/echo time/inversion recovery,
6,960/79/150 msec; acquisition matrix, 176×115; and reconstruction
matrix, 256; field of view: Right/left, 530 mm; anterior/posterior,
349 mm; and feet/head, 226 mm; slice thickness, 6 mm; size of
reconstructed voxel, 2.07×2.08×6 mm3; 4 averages. One
radiologist and one gastroenterologist analyzed the DWIBS/T2
images. To rule out T2 shine-through or differentiate malignant
lesions from non-malignant causes of restricted diffusion, a
‘positive apparent diffusion (ADC) map’ was determined as a
decreased signal on the ADC coefficient with ADC reduction
(15).
 | Table I.Pulse sequences used in the present
study. |
Table I.
Pulse sequences used in the present
study.
| Parameters | T1-weighted
image | T2-weighted
image | DWI (DWIBS/T2) |
|---|
| Echo | GRE | Single-shot SE | EPI SE |
| TR (msec) | Shortest | 1,000 | 11,250 |
| TE (msec) | First: 2.3
(out-phase), second, 4.6 (in-phase) | 90 | 83 |
| Flip angle (°) | 75 | 90 | 90 |
| NSA | 1 | 1 | 4 |
| Slice thickness
(mm) | 8 | 8 | 5 |
| Slice gap | 1 | 1 | 0 |
| Fat saturation | None | None | SPAIR |
| Phase encoding
direction |
Posterior-anterior |
Posterior-anterior |
Posterior-anterior |
Upper gastrointestinal tract
endoscopy, EUS and colonoscopy
The endoscopic devices used in the upper tract were
the GIF-N260H, GIF-XP260NS, GIF-PG260, GIF-XQ260 and GIF-Q260
(Olympus, Tokyo, Japan). EUS was performed using GF-UCT260
(Olympus). The devices used for colonoscopy were the CF-Q260 and
PCF-Q260AI (Olympus).
Statistical analysis
One-way analysis of variance or the Chi-squared test
were applied using JMP 0.0.2 software (SAS Institute, Cary, NC,
USA). Values are expressed as the mean ± standard deviation.
Results
Patient characteristics
The patient details and diagnoses are summarized in
Table II. Gastrointestinal tract
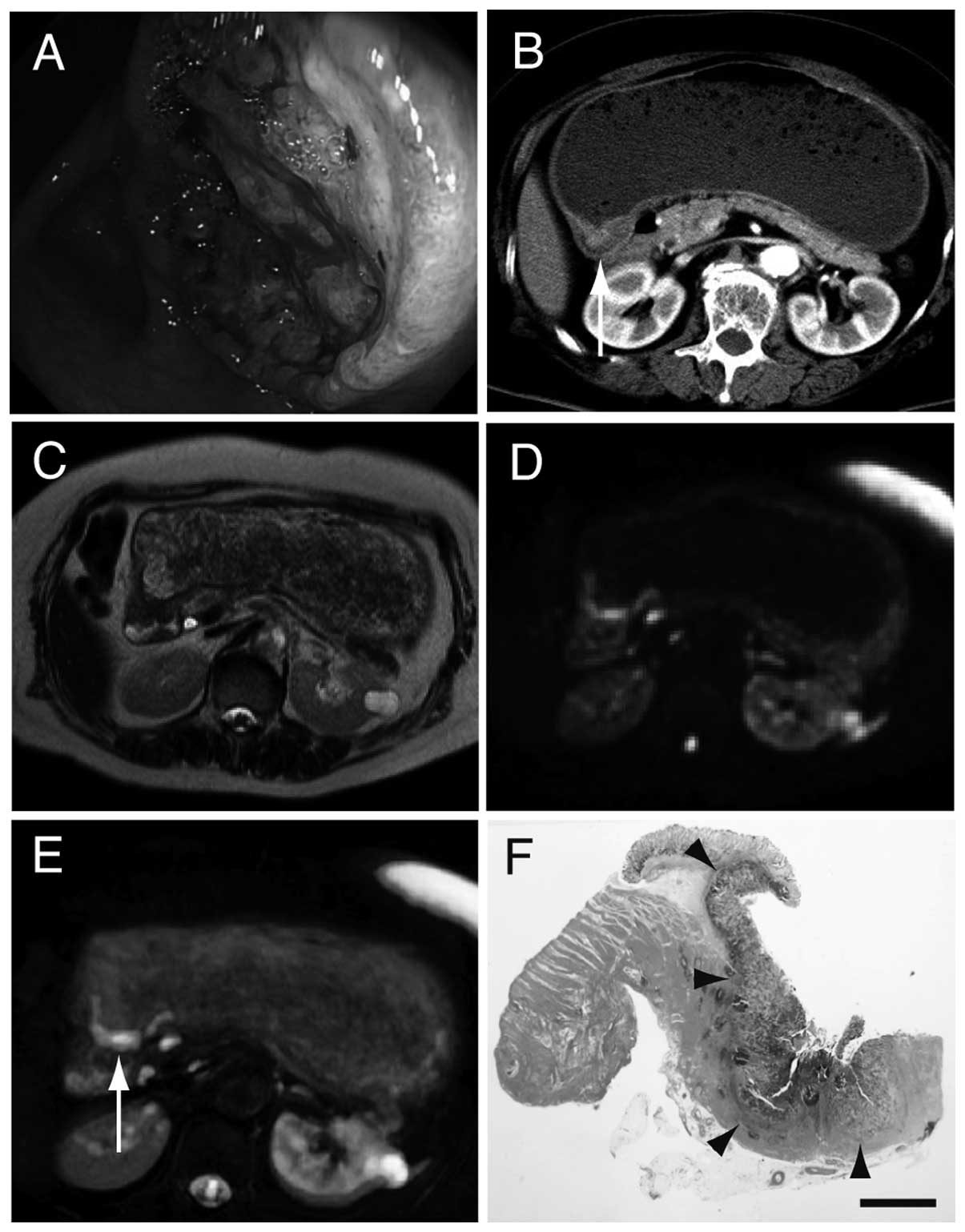
cancers were initially diagnosed by endoscopy (Fig. 1A). T staging was performed based on
CE-CT and other diagnostic imaging techniques (Fig. 1B). Colon cancer was detected by T1WI
as it was a polyp protruding into the lumen. T2WI was positive in
one patient with gastric cancer and one patient with colon cancer.
A thickened wall was identified by T1WI and T2WI in some cases;
however, it was difficult to diagnose the other lesions as
cancerous, as their intensities were identical to that of the
surrounding tissues. A total of 12 patients were detected with DWI
or DWIBS/T2. DWI and DWIBS/T2 were more sensitive compared with
T2WI alone (Fig. 1C and D). DWI and
DWIBS/T2 exhibited a significant contrast and had the same
sensitivity. With DWIBS/T2 it was easier to analyze the strong
positive signal in an anatomical context (Fig. 1E). Three patients with gastric cancer
who were negative on DWIBS/T2, were found to be stage T1a and 1
patient with duodenal cancer who was negative on DWIBS/T2, was
staged as Tis. The mechanism underlying the negative results on
DWIBS/T2 is intriguing. The shape of the positive signal on
DWIBS/T2 was consistent with that of the surgical specimen
(Fig. 1F).
 | Table II.List of patients diagnosed with
cancer. |
Table II.
List of patients diagnosed with
cancer.
| Patient number | Diagnosis | T stagea | Depth of
invasion | Diameter (cm) | T1W | T2W | DWI | DWIBS/T2 |
|---|
| 1 | Esophageal
cancer | 3 | NA | NA | (−) | (−) | (+) | (+) |
| 2 | Gastric cancer | 1a | M | 1.5 | (−) | (−) | (−) | (−) |
| 3 | Gastric cancer | 1a | M | 1.3 | (−) | (−) | (−) | (−) |
| 4 | Gastric cancer | 1a | M | 1.8 | (−) | (−) | (−) | (−) |
| 5 | Gastric cancer | 2 | MP | 4 | (−) | (−) | (+) | (+) |
| 6 | Gastric cancer | 3 | SS | 1.3 | (−) | (−) | (+) | (+) |
| 7 | Gastric cancer | 4 | NA | NA | (−) | (−) | (+) | (+) |
| 8 | Gastric cancer | 3 | NA | NA | (−) | (−) | (+) | (+) |
| 9 | Gastric cancer | 3 | NA | NA | (−) | (+) | (+) | (+) |
| 10 | Gastric cancer | 3 | NA | NA | (−) | (−) | (+) | (+) |
| 11 | Gastric cancer | 3 | NA | NA | (−) | (−) | (+) | (+) |
| 12 | Gastric cancer | 4b | SS | 5 | (−) | (−) | (+) | (+) |
| 13 | Gastric cancer | 3a | MP | 6 | (−) | (−) | (+) | (+) |
| 14 | Duodenal
cancer | is | NA | NA | (−) | (−) | (−) | (−) |
| 15 | Duodenal
cancer | 2 | NA | NA | (−) | (−) | (+) | (+) |
| 16 | Colon
cancerb | is | M | 1.5 | (+) | (+) | (+) | (+) |
Association of detectability with T
stage
Subsequently, we focused on T staging and the
association between tumor detectability with DWIBS/T2 and T stage
was analyzed (Table III). All
cancers staged >T2 were detectable by DWIBS/T2 and all cancers
staged <T1 were not, clearly indicating that advanced cancer
stage is significantly associated with its detectability with
DWIBS/T2 (P<0.0001).
 | Table III.Correlation between tumor
detectability by DWIBS/T2 and T stage. |
Table III.
Correlation between tumor
detectability by DWIBS/T2 and T stage.
|
| T
stagea |
|
|---|
|
|
|
|
|---|
| Detection | >T2 | <T1 | Total |
|---|
| (+) | 12 | 0 | 12 |
| (−) | 0 | 4 | 4 |
| Total | 12 | 4 | 16 |
Association of detectability with
depth of invasion
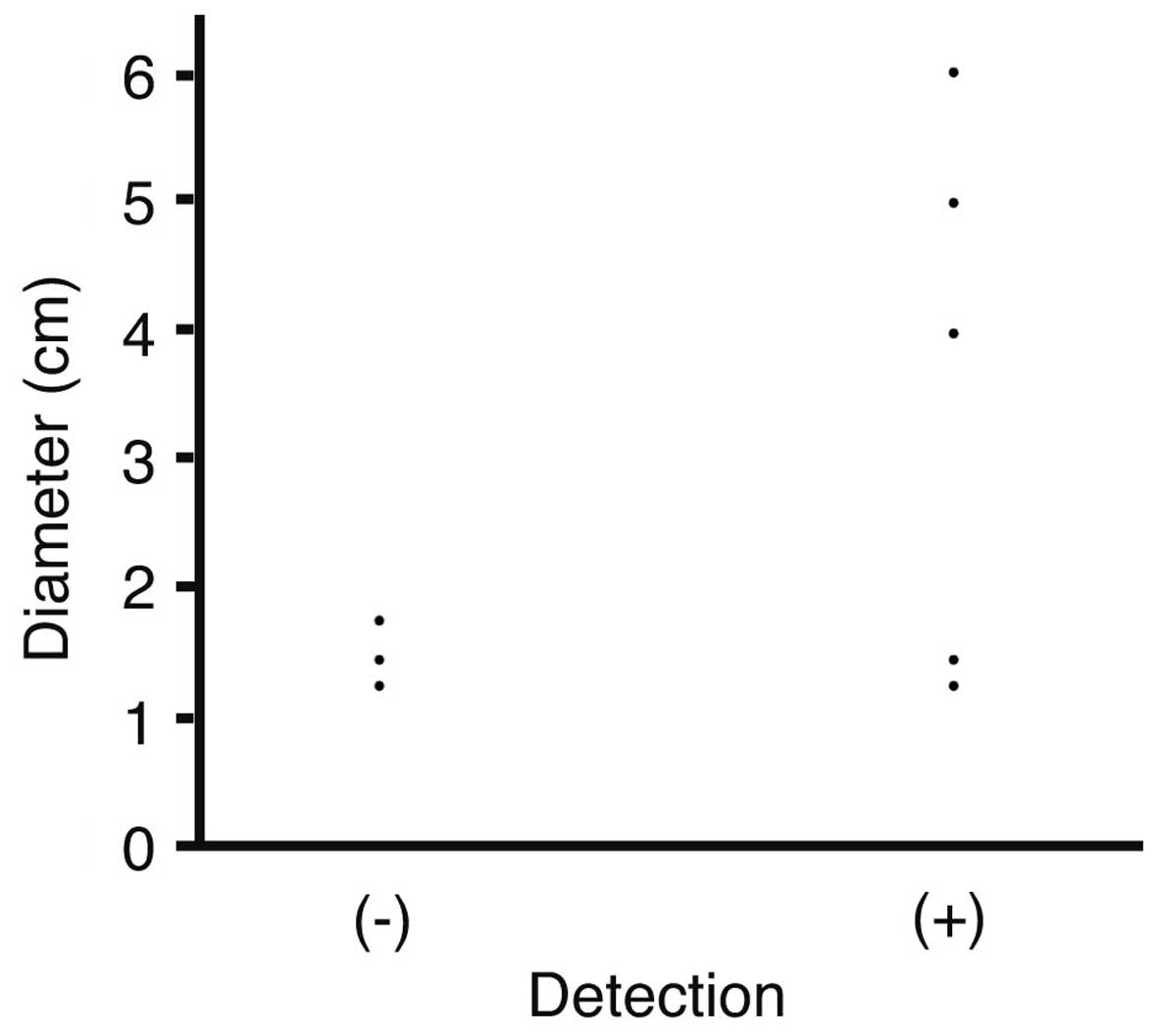
The association between the tumor diameter and
detectability was next analyzed. Diameters were plotted against
detection with DWIBS/T2 (Fig. 2). The
mean diameter of tumors not detected by DWIBS/T2 was 1.53±0.25 cm,
while that of detected tumors was 3.63±1.88 cm; however, the
difference was not statistically significant (P=0.1053).
Furthermore, the association of depth of invasion of
the tumors with their detectability by DWIBS/T2 was assessed
(Table IV). All 5 cancers that had
invaded beyond the muscularis propria were detected by DWIBS/T2,
whereas 3 cases that had not invaded the mucosa were not detected.
The depth of invasion was significantly associated with
detectability by DWIBS/T2 (P=0.0476). Colon cancer was positive on
DWIBS/T2, although was confined in the mucosa; the cancer was
originally a colon polyp with a diameter of 1.5 cm.
 | Table IV.Association between tumor
detectability by DWIBS/T2 and depth of invasion. |
Table IV.
Association between tumor
detectability by DWIBS/T2 and depth of invasion.
|
| Depth of
invasion |
|
|---|
|
|
|
|
|---|
| Detection | >MP | M | Total |
|---|
| (+) | 5 | 1a | 5 |
| (−) | 0 | 3 | 4 |
| Total | 5 | 4 | 9 |
Discussion
Until recently, DWI or DWIBS with a 1.5-Tesla
scanner was considered to be unsuitable for imaging of abdominal
organs due to respiratory movement (16–18).
However, the protocol of acquiring images has improved with the use
of a respiratory trigger (6). In the
present study, all gastric and duodenal cancers staged >T2 were
detectable by DWI and DWIBS/T2 (19).
DWI and DWIBS/T2 exhibited a strong signal and contrast against the
surrounding tissues. For this reason, DWI and DWIBS/T2 had better
sensitivity when compared with T2WI alone. Unlike endoscopy,
DWIBS/T2 may be useful for evaluating the extent and depth of
invasion of gastric cancer (20–22). By
contrast, all gastric and duodenal cancers exhibiting invasion of
<T1 were not detected by DWIBS/T2, indicating that T stage
affected tumor detectability by DWIBS/T2. In particular, Borrmann 4
gastric cancer exhibits a thickened wall, referred to as ‘sandwich
sign’ (23). Our findings suggested
that DWIBS/T2 may add diagnostic information to the process of
T-staging (20–22,24).
T staging is performed based on the depth of
invasion regarding gastrointestinal tract cancers. The present
study revealed that cancers confined within the mucosa were not
detected by DWIBS/T2. One exception was a case of colon cancer; the
patient presented with a colon polyp and underwent endoscopic
mucosal resection. The polyp was 1.5 cm in diameter and protruded
into the lumen. It was hypothesized that the polyp was of
sufficient size to be detectable by DWIBS/T2, even though the
cancer had only invaded the mucosa. Of note, all other cancers that
were not detectable by DWIBS/T2 were flat. Cancers confined within
the mucosa may be positive on DWIBS/T2 upon reaching a certain
volume. High-spatial resolution MRI is able to detect gastric
cancer within the mucosa (22).
However, this technique is currently not applied.
One limitation of the present study was the small
number of patients. Further studies including more colon and
duodenal cancer patients are required to confirm our findings.
Another limitation was that tumor invasion of the muscularis
propria (PM), subserosa (SS) and serosal exposure (SE) was not
analyzed. In future studies, the possibility to differentiate
between PM, SS and SE invasion with DWIBS/T2 compared with
endoscopic ultrasound should be addressed (25).
In conclusion, DWIBS/T2 was able to identify
gastrointestinal cancers staged as >T2 or invading beyond the
muscularis propria.
References
|
1
|
Allum WH, Blazeby JM, Griffin SM,
Cunningham D, Jankowski JA and Wong R: Association of Upper
Gastrointestinal Surgeons of Great Britain and Ireland, the British
Society of Gastroenterology and the British Association of Surgical
Oncology: Guidelines for the management of oesophageal and gastric
cancer. Gut. 60:1449–1472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Labianca R, Nordlinger B, Beretta GD,
Mosconi S, Mandalà M, Cervantes A and Arnold D: ESMO Guidelines
Working Group: Early colon cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
24(Suppl 6): vi64–vi72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Didden P, Spaander MC, Wijnhoven BP,
Kuipers EJ and Bruno MJ: Improving the quality of pretreatment
staging in patients with esophageal carcinoma-a fast track study.
Acta Oncol. 51:362–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Umeoka S, Koyama T, Togashi K, Saga T,
Watanabe G, Shimada Y and Imamura M: Esophageal cancer: Evaluation
with triple-phase dynamic CT-initial experience. Radiology.
239:777–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi JI, Joo I and Lee JM:
State-of-the-art preoperative staging of gastric cancer by MDCT and
magnetic resonance imaging. World J Gastroenterol. 20:4546–4557.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahara T, Imai Y, Yamashita T, Yasuda S,
Nasu S and Van Cauteren M: Diffusion weighted whole body imaging
with background body signal suppression (DWIBS): Technical
improvement using free breathing, STIR and high resolution 3D
display. Radiat Med. 22:275–282. 2004.PubMed/NCBI
|
|
7
|
Sehy JV, Ackerman JJ and Neil JJ: Apparent
diffusion of water, ions, and small molecules in the Xenopus oocyte
is consistent with Brownian displacement. Magn Reson Med. 48:42–51.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koike N, Cho A, Nasu K, Seto K, Nagaya S,
Ohshima Y and Ohkohchi N: Role of diffusion-weighted magnetic
resonance imaging in the differential diagnosis of focal hepatic
lesions. World J Gastroenterol. 15:5805–5812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwee TC, Takahara T, Ochiai R, Nievelstein
RA and Luijten PR: Diffusion-weighted whole-body imaging with
background body signal suppression (DWIBS): Features and potential
applications in oncology. Eur Radiol. 18:1937–1952. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohno Y, Koyama H, Onishi Y, Takenaka D,
Nogami M, Yoshikawa T, Matsumoto S, Kotani Y and Sugimura K:
Non-small cell lung cancer: Whole-body MR examination for M-stage
assessment-utility for whole-body diffusion-weighted imaging
compared with integrated FDG PET/CT. Radiology. 248:643–654. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fischer MA, Nanz D, Hany T, Reiner CS,
Stolzmann P, Donati OF, Breitenstein S, Schneider P, Weishaupt D,
von Schulthess GK and Scheffel H: Diagnostic accuracy of whole-body
MRI/DWI image fusion for detection of malignant tumours: A
comparison with PET/CT. Eur Radiol. 21:246–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sommer G, Wiese M, Winter L, Lenz C,
Klarhöfer M, Forrer F, Lardinois D and Bremerich J: Preoperative
staging of non-small-cell lung cancer: Comparison of whole-body
diffusion-weighted magnetic resonance imaging and
18F-fluorodeoxyglucose-positron emission tomography/computed
tomography. Eur Radiol. 22:2859–2867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nechifor-Boilă IA, Bancu S, Buruian M,
Charlot M, Decaussin-Petrucci M, Krauth JS, Nechifor-Boilă AC and
Borda A: Diffusion weighted imaging with background body signal
suppression/T2 image fusion in magnetic resonance mammography for
breast cancer diagnosis. Chirurgia (Bucur). 108:199–205.
2013.PubMed/NCBI
|
|
14
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Miller FH, Chen ZE, Merrick L,
Mortele KJ, Hoff FL, Hammond NA, Yaghmai V and Nikolaidis P:
Diffusion-weighted MR imaging of solid and cystic lesions of the
pancreas. Radiographics. 31:E47–E64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mürtz P, Krautmacher C, Träber F, Gieseke
J, Schild HH and Willinek WA: Diffusion-weighted whole-body MR
imaging with background body signal suppression: A feasibility
study at 3.0 Tesla. Eur Radiol. 17:3031–3037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caivano R, Rabasco P, Lotumolo A,
D'Antuono F, Zandolino A, Villonio A, Macarini L, Guglielmi G,
Salvatore M and Cammarota A: Gastric cancer: The role of diffusion
weighted imaging in the preoperative staging. Cancer Invest.
32:184–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Cobelli F, Giganti F, Orsenigo E,
Cellina M, Esposito A, Agostini G, Albarello L, Mazza E, Ambrosi A,
Socci C, et al: Apparent diffusion coefficient modifications in
assessing gastro-oesophageal cancer response to neoadjuvant
treatment: Comparison with tumour regression grade at histology.
Eur Radiol. 23:2165–2174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huo X, Yuan K, Shen Y, Li M, Wang Q, Xing
L and Shi G: Clinical value of magnetic resonance imaging in
preoperative T staging of gastric cancer and postoperative
pathological diagnosis. Oncol Lett. 8:275–280. 2014.PubMed/NCBI
|
|
20
|
Mocellin S, Marchet A and Nitti D: EUS for
the staging of gastric cancer: A meta-analysis. Gastrointest
Endosc. 73:1122–1134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bohle W, Scheidig A and Zoller WG:
Endosonographic tumor staging for treatment decision in resectable
gastric cancer. J Gastrointestin Liver Dis. 20:135–139.
2011.PubMed/NCBI
|
|
22
|
Yamada I, Saito N, Takeshita K, Yoshino N,
Tetsumura A, Kumagai J and Shibuya H: Early gastric carcinoma:
Evaluation with high-spatial-resolution MR imaging in vitro.
Radiology. 220:115–121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang XP, Tang L, Sun YS, Li ZY, Ji JF, Li
XT, Liu YQ and Wu Q: Sandwich sign of Borrmann type 4 gastric
cancer on diffusion-weighted magnetic resonance imaging. Eur J
Radiol. 81:2481–2486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu S, He J, Guan W, Li Q, Yu H and Zhou
Z, Bao S and Zhou Z: Added value of diffusion-weighted MR imaging
to T2-weighted and dynamic contrast-enhanced MR imaging in T
staging of gastric cancer. Clin Imaging. 38:122–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei C, Huang L, Wang Y and Huang Y and
Huang Y: Comparison of MRI and endoscope ultrasound detection in
preoperative T/N staging of gastric cancer. Mol Clin Oncol.
1:699–702. 2013.PubMed/NCBI
|