Background
Lung cancer is considered to be one of the leading
causes of mortality worldwide in the 21st century. Millions of
individuals die from lung cancer annually (1). Mortality caused by lung cancer ranks
first among other cancer types, regardless of gender. Among lung
cancer cases, non-small cell lung cancer (NSCLC) accounts for
80–85% of the total. Although medical treatments for NSCLC have
greatly progressed, this cancer type continues to be associated
with one of the most malignant types of tumour, with the worst
post-operative therapeutic effects (2). With the help of early diagnosis and
treatment, the five-year survival rate of patients with NSCLC has
increased (3). Therefore, researchers
have aimed to develop novel reliable and atraumatic methods to
diagnose and assess lung cancer. Similarly, the present study aimed
to develop a novel method that may be used to diagnose and evaluate
lung cancer.
As an indication of airway canceration, exhaled
breath condensate (EBC) comprises the liquid and volatile mixture
secreted by the lower respiratory tract mucosa (4). EBC collection techniques exhibit several
advantages, including non-invasiveness, simplicity in their
execution and high repeatability (5,6). The
detection of tumour markers in EBC may be a novel approach to
diagnose lung cancer in the early stages and in screens of
high-risk groups (7–10). In the present study, cancer embryo
antigen (CEA) and endothelin-1 (ET-1) were detected in the EBC of
133 patients with NSCLC.
Subjects and methods
Study subjects
A total of 143 patients were diagnosed with squamous
cell carcinoma and adenocarcinoma, who received bronchoscopy, lung
biopsy and open chest surgery in our hospital from February 2011 to
October 2014. The patients with severe heart disease and damaged
liver or kidney functions, and those who failed to cooperate during
the study, were excluded. Of the 143 subjects, 75 suffered from
adenocarcinoma and 68 endured squamous cell carcinoma. According to
the seventh version of the lung cancer tumour-node-metastasis
staging standard provided by the Union for International Cancer
Control in 2009 (11), 15 cases were
in stage I, 29 cases were in stage II, 59 cases were in stage III
and 40 cases were in stage IV. Moreover, 119 healthy volunteers
were included in the normal control group. No statistically
significant differences were identified in terms of the age, gender
or smoking history of the participants.
Sample collection
EBC was collected using a HAAK EK20 EcoScreen (Eric
Jaeger, Friedberg, Germany). The subjects wore nose-clips and
maintained eupnoea for 20 min by biting mouthparts. This method was
used to collect 1–3 ml condensate from each subject; the obtained
condensate was subsequently preserved at −70°C. Simultaneously, 3
ml fasting venous blood was drawn early in the morning, and the
serum was extracted. The samples were allowed to coagulate at room
temperature for 30 min, and subsequently were centrifuged at 2,500
g for 20 min. The samples were preserved at −20°C, and the test was
conducted within a week.
Testing method
Chemiluminescence microparticle immunoassays were
performed using a CEA kit (Abbott Laboratories, Abbott Park, IL,
USA). An enzyme-linked immunosorbent assay (ELISA) was performed
using an ET-1 kit (R&D Systems, Inc., Minneapolis, MN, USA).
The experiment was performed in accordance with the manufacturer's
protocol.
Statistical analysis
Data were statistically analysed using SPSS 13.0
software (SPSS, Inc., Chicago,. IL, USA). Measured data were in
accordance with the normal distribution of the mean ± standard
deviation (x̅ ± s) under a normal distribution test. The two
samples were compared using a t-test, and the measured data were
subjected to a χ2 test. The correlation between CEA and
ET-1 in the EBC and serum was determined through correlational
analysis. Receiver operating characteristic (‘ROC’) curve analysis
was performed to determine the specificity and sensitivity of the
method to diagnose lung cancer. P<0.05 was considered to
indicate a statistically significant value.
Results
The levels of CEA and ET-1 in the serum and EBC
samples were compared between the NSCLC group and the healthy group
(Table I), revealing that the CEA
levels in the EBC and serum of the lung cancer group were
significantly higher compared with those of the normal control
group (P<0.01). The levels of CEA and ET-1 in the serum and EBC
samples were also compared between the two pathological lung cancer
types investigated (adenocarcinoma and squamous cell carcinoma)
(Table II). This analysis determined
that the level of CEA in the EBC and serum of the patients with
adenocarcinoma was significantly higher compared with that of the
patients with squamous cell carcinoma.
 | Table I.Comparison of the CEA and ET-1 levels
in the serum and EBC of the two groups (x̅ ± s). |
Table I.
Comparison of the CEA and ET-1 levels
in the serum and EBC of the two groups (x̅ ± s).
|
|
| EBC | Serum |
|---|
|
|
|
|
|
|---|
| Parameter | N | CEA (mg/l) | ET-1 (ng/l) | CEA (mg/l) | ET-1 (ng/l) |
|---|
| Subject |
|
|
NSCLC | 143 | 2.96±1.54 | 19.68±7.41 | 14.52±7.48 | 63.30±20.04 |
|
Healthy | 119 | 0.82±0.42 |
7.35±3.15 |
3.59±2.07 | 46.51±15.16 |
| t-test |
| 14.753 | 16.913 | 15.455 | 7.520 |
| P-value |
| <0.01 | <0.01 | <0.01 | <0.01 |
 | Table II.Association between the levels of CEA
and ET-1 and the pathological type (x̅ ± s). |
Table II.
Association between the levels of CEA
and ET-1 and the pathological type (x̅ ± s).
|
|
| EBC | Serum |
|---|
|
|
|
|
|
|---|
| Parameter | N | CEA (mg/l) | ET-1 (ng/l) | CEA (mg/l) | ET-1 (ng/l) |
|---|
| Cancer type |
|
|
Adenocarcinoma | 75 | 3.99±1.09 | 20.07±7.24 | 19.27±5.87 | 64.96±21.87 |
| Squamous
cell | 68 | 1.82±1.08 | 19.25±7.63 |
9.28±5.23 | 61.46±17.80 |
|
carcinoma |
|
| t-test |
| 11.946 | 0.658 | 3.977 | 1.044 |
| P-value |
| <0.01 | >0.05 | <0.01 | >0.05 |
The correlation of the levels of CEA and ET-1 with
lung cancer staging was subsequently determined (Table III). These results revealed that the
levels of CEA in the EBC and serum of the patients with lung cancer
stages III and IV were significantly higher compared with those of
the patients with lung cancer stage I and II (P<0.01).
 | Table III.Relationship of the CEA and ET-1
levels with lung cancer staging (x̅ ± s). |
Table III.
Relationship of the CEA and ET-1
levels with lung cancer staging (x̅ ± s).
|
|
| EBC | Serum |
|---|
|
|
|
|
|
|---|
| Parameter | N | CEA (mg/l) | ET-1 (ng/l) | CEA (mg/l) | ET-1 (ng/l) |
|---|
| Stage
I/IIa | 44 | 1.53±1.18 | 15.29±8.22 |
8.48±5.29 | 53.73±21.10 |
| Stage III/IV | 99 | 3.59±1.22 | 21.63±6.12 | 17.20±6.72 | 67.55±18.09 |
| t-test |
| 9.410 | 5.125 | 7.623 | 4.000 |
| P-value |
| <0.01 | <0.01 | <0.01 | <0.01 |
The correlation between the levels of CEA and ET-1
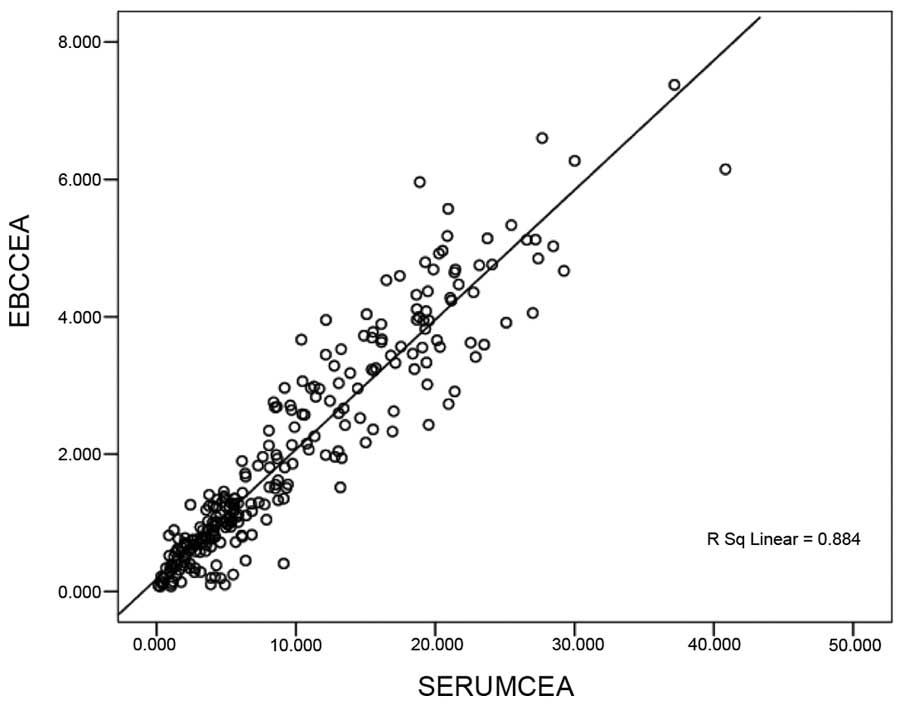
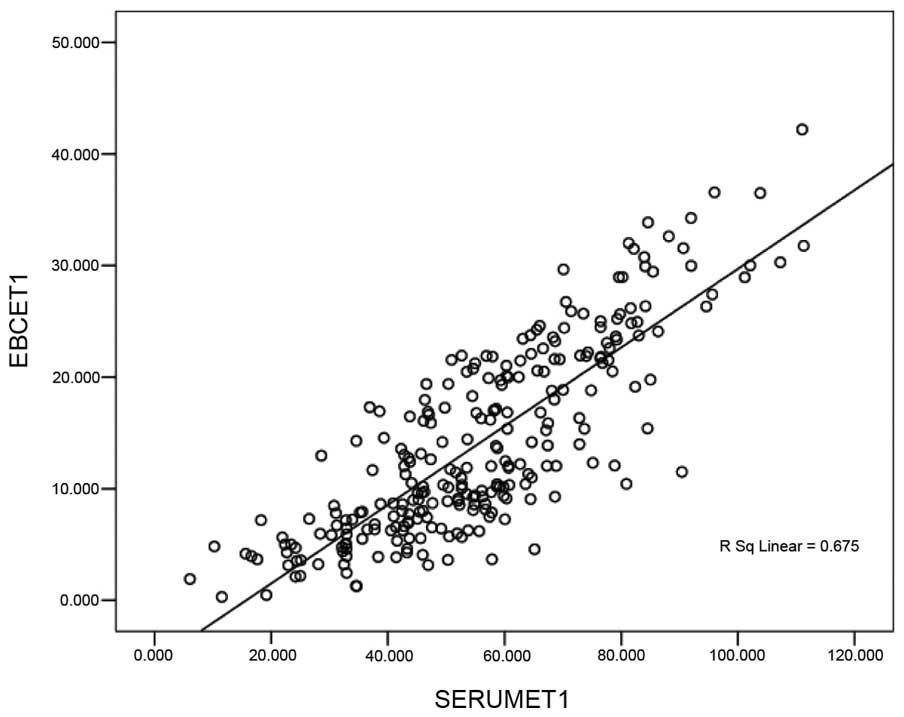
in the EBC and serum is shown in Figs.
1 and 2. These results
demonstrated a positive linear correlation between the levels of
CEA identified in the EBC and in serum CEA (Fig. 1); a similar result was identified with
the level of ET-1 in the EBC and the serum: The correlation
coefficients (r) were calculated to be 0.884 and 0.675,
respectively (P<0.05).
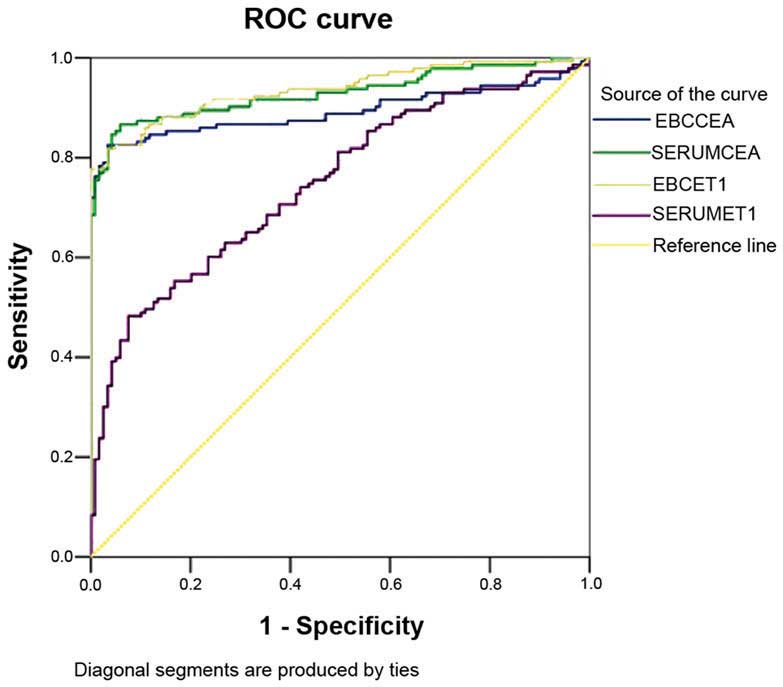
Subsequently, the sensitivity and specificity of the
levels of CEA and ET-1 were analysed to diagnose lung cancer
(Fig. 3 and Table IV).
 | Table IV.Specificity and sensitivity of CEA and
ET-1 in the EBC and serum to diagnose lung cancer. |
Table IV.
Specificity and sensitivity of CEA and
ET-1 in the EBC and serum to diagnose lung cancer.
| Parameter | Area under the ROC
curve | Critical value | Sensitivity (%) | Specificity
(%) |
|---|
| EBC CEA | 0.893 | 1.505
mg/l | 82.5 | 96.6 |
| Serum CEA | 0.929 | 7.063
mg/l | 84.6 | 95.8 |
| EBC ET-1 | 0.936 | 12.681 ng/l | 81.8 | 96.6 |
| Serum ET-1 | 0.748 | 65.334 ng/l | 48.0 | 92.4 |
Discussion
EBC provides a novel tool to detect the biochemical
constituents of the respiratory tract; EBC does not interfere with
the physiological or pathological processes of the respiratory
tract (12). Collection of the EBC is
completely atraumatic, and does not harm the bronchial mucosa.
Furthermore, EBC is directly collected from the airways, and
dilution is impossible. Therefore, the results from such a study
may be considered to be reliable and highly repeatable (13). Thus far, studies have focused on
markers of inflammation in the EBC, including nitric oxide, carbon
monoxide, 8-iso-prostaglandin and leukotriene (14–16).
However, researchers have rarely investigated EBC tumour markers
(17,18). Figs. 1
and 2 revealed a positive linear
correlation between the levels of CEA determined in the EBC and in
the serum. A similar correlation was also observed between the
levels of ET-1 in the EBC and the serum. The correlation
coefficients were 0.884 and 0.675, respectively (P<0.05). These
results indicated that this reliable and atraumatic EBC collection
method may be used to effectively detect the levels of CEA and ET-1
in patients. In addition, this method also helped in the auxiliary
diagnosis of NSCLC.
CEA is one of the tumour markers used to diagnose
NSCLC. Table I indicated that the
levels of CEA in the EBC and serum of the lung cancer group were
significantly higher compared with those of the normal control
group (P<0.01); this result is consistent with the findings of a
previous study (19). In addition,
the CEA and ET-1 levels in the EBC exhibited sensitivity (82.5% for
EBC CEA; 81.8% for EBC ET-1) and specificity (96.6% for both EBC
CEA and EBC ET-1) to diagnose lung cancer; thus, the level of CEA
in the EBC may help in the auxiliary diagnosis of NSCLC. EBC CEA
serves as a supplementary diagnostic index for lung cancers that
cannot be specifically diagnosed through traumatic detection, for
example, via bronchoscopy.
CEA is considered to be the preferred indicator of
lung adenocarcinoma (20). Table II shows that the positive expression
rates of CEA in squamous cell carcinoma and adenocarcinoma were
42.9 and 73.3%, respectively. The level of CEA in the EBC and serum
of adenocarcinoma patients was higher compared with that of
squamous cell carcinoma patients: This finding is consistent with
those described in other studies (19,21). This
finding also revealed that the level of CEA in the EBC is valuable
for the diagnosis of pathological lung cancer types. This analysis
indicated that the levels of CEA in the EBC and serum of patients
with lung cancer in stages III and IV were higher compared with
those of patients with lung cancer in stages I and II (Table III). The level of CEA is likely to
increase as the disease progresses (21), and this pattern revealed that the
regular monitoring of EBC CEA may contribute to the assessment of
lung cancer development. Regular monitoring may also serve as a
guide for clinical treatments (22).
ET-1 is an active peptide of 21 amino acid residues
from vascular endothelial cells. ET-1 is also a strong accelerant
of cell mitosis. ET-1 is able to promote tumour cell proliferation
and capillary formation (23). The
highest content of ET-1 is to be found in the kidney, followed by
the lungs; thus, the cells in these organs are conducive to DNA
synthesis and proliferation. Previous studies have revealed that an
increase in the levels of endothelin-converting enzyme results in
an increase in ET-1 secretory volume. The final process of the
endothelin-converting enzyme, which catalyses endothelin synthesis,
is closely associated with the biological behaviour of several
malignant tumours. However, only a few studies have focused on ET-1
in EBC. An ELISA was performed in the present study to detect ET-1
in the EBC and serum. This method confirmed that the levels of ET-1
in the EBC and serum of the NSCLC group were higher compared with
those in the normal control group (Table
I). The specificity and sensitivity of the method to detect
ET-1 in the EBC were higher compared with those to detect ET-1 in
the serum; therefore, detection of ET-1 in the EBC is of greater
importance for the auxiliary diagnosis of NSCLC with respect to the
detection of ET-1 in the serum.
Table III also
revealed that the ET-1 levels in the EBC and serum of the patients
with lung cancer in stages III and IV were higher compared with
those of patients with lung cancer in stages I and II. The level of
ET-1 increased as the disease progresses; this finding is
consistent with that of Carpagnano et al (24) and Boldrini et al (25). This tendency indicated that ET-1
induces the growth and metastasis of lung cancer. The presence of a
high level of ET-1 would correspond to a high likelihood of
metastasis and the worst post-operative effects. ET-1 in the EBC
may be used as an index to monitor lung cancer metastasis and to
assess the effect of surgery. This is a possibility, partly due to
the fact that ET-1 is a hypoxia-inducible angiogenic growth factor.
ET-1 promotes angiogenesis in lung cancer, DNA synthesis and cell
proliferation; thus, ET-1 contributes to the growth and metastasis
of lung tumours.
In conclusion, CEA and ET-1 may be detected in the
EBC of patients with NSCLC, and these parameters are of great
importance in the early diagnosis, monitoring, pathological type
determination and prognosis of NSCLC. As a novel approach to
investigate patients with NSCLC, the collection of EBC is easy,
atraumatic, repeatable and comparable. Therefore, this technique
should be further promoted.
Acknowledgements
The present study was supported by a Clinical Key
Speciality Project of China.
References
|
1
|
Eberini I, Gianazza E, Pastorino U and
Sirtori C: Assessment of individual lung cancer risk by the
proteomic analysis of exhaled breath condensate. Expert Opin Med
Diagn. 2:1309–1315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pillai RN and Ramalingam SS: Advances in
the diagnosis and treatment of non-small cell lung cancer. Mol
Cancer Ther. 13:557–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Padda SK, Burt BM, Trakul N and Wakelee
HA: Early-stage non-small cell lung cancer: Surgery, stereotactic
radiosurgery and individualized adjuvant therapy. Semin Oncol.
41:40–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalaveris E, Kerenidi T,
Katsabeki-Katsafli A, Kiropoulos T, Tanou K, Gourgoulianis KI and
Kostikas K: VEGF, TNF-alpha and 8-isoprostane levels in exhaled
breath condensate and serum of patients with lung cancer. Lung
Cancer. 64:219–225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ciebiada M, Górski P and Antczak A:
Eicosanoids in exhaled breath condensate and bronchoalveolar lavage
fluid of patients with primary lung cancer. Dis Markers.
32:329–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antus B and Barta I: Exhaled breath
condensate pH in patients with lung cancer. Lung Cancer.
75:178–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan HP, Lewis C and Thomas PS: Exhaled
breath analysis: Novel approach for early detection of lung cancer.
Lung Cancer. 63:164–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mozzoni P, Banda I, Goldoni M, Corradi M,
Tiseo M, Acampa O, Balestra V, Ampollini L, Casalini A, Carbognani
P and Mutti A: Plasma and EBC microRNAs as early biomarkers of
non-small-cell lung cancer. Biomarkers. 18:679–686. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stathopoulos D, Loukides S and Syrigos K:
8-Isoprostane in exhaled breath condensate of patients with
non-small cell lung cancer: The effect of chemotherapy. Anticancer
Res. 34:5143–5145. 2014.PubMed/NCBI
|
|
10
|
Brussino L, Culla B, Bucca C, Giobbe R,
Boita M, Isaia G, Heffler E, Oliaro A, Filosso P and Rolla G:
Inflammatory cytokines and VEGF measured in exhaled breath
condensate are correlated with tumor mass in non-small cell lung
cancer. J Breath Res. 8:0271102014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dassanayake DL, Muthunayake TM,
Senevirathna KH and Siribaddana A: Staging of lung cancer in a
tertiary care setting in Sri Lanka, using TNM 7th edition. A
comparison against TNM6. BMC Res Notes. 5:1432012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fumagalli M, Ferrari F, Luisetti M, Stolk
J, Hiemstra PS, Capuano D, Viglio S, Fregonese L, Cerveri I, Corana
F, et al: Profiling the proteome of exhaled breath condensate in
healthy smokers and COPD patients by LC-MS/MS. Int J Mol Sci.
13:13894–13910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu HC, Lu MC, Lin YC, Wu TC, Hsu JY, Jan
MS and Chen CM: Differences in IL-8 in serum and exhaled breath
condensate from patients with exacerbated COPD or asthma attacks. J
Formos Med Assoc. 113:908–914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee AL, Button BM, Denehy L, Roberts S,
Bamford T, Mu FT, Mifsud N, Stirling R and Wilson JW: Exhaled
breath condensate pepsin: Potential noninvasive test for
gastroesophageal reflux in COPD and bronchiectasis. Respir Care.
60:244–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corhay JL, Moermans C, Henket M, Nguyen
Dang D, Duysinx B and Louis R: Increased of exhaled breath
condensate neutrophil chemotaxis in acute exacerbation of COPD.
Respir Res. 15:1152014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stefanska J, Sarniak A, Wlodarczyk A,
Sokolowska M, Doniec Z, Bialasiewicz P, Nowak D and Pawliczak R:
Hydrogen peroxide and nitrite reduction in exhaled breath
condensate of COPD patients. Pulm Pharmacol Ther. 25:343–348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao P, Chen JR, Zhou F, Lu CX, Yang Q,
Tao GH, Tao YJ and Chen JL: Methylation of P16 in exhaled breath
condensate for diagnosis of non-small cell lung cancer. Lung
Cancer. 83:56–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gessner C, Kuhn H, Toepfer K,
Hammerschmidt S, Schauer J and Wirtz H: Detection of p53 gene
mutations in exhaled breath condensate of non-small cell lung
cancer patients. Lung Cancer. 43:215–222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou Y, Wang L, Zhao C, Hu Y, Xu S, Ying K,
Wang P and Chen X: CEA, SCC and NSE levels in exhaled breath
condensate-possible markers for early detection of lung cancer. J
Breath Res. 7:0471012013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okamura K, Takayama K, Izumi M, Harada T,
Furuyama K and Nakanishi Y: Diagnostic value of CEA and CYFRA 21-1
tumor markers in primary lung cancer. Lung Cancer. 80:45–49. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cedrés S, Nuñez I, Longo M, Martinez P,
Checa E, Torrejón D and Felip E: Serum tumor markers CEA, CYFRA21-1
and CA-125 are associated with worse prognosis in advanced
non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 12:172–179.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grunnet M and Sorensen JB:
Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung
Cancer. 76:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang WM, Zhou J and Ye QJ: Endothelin-1
enhances proliferation of lung cancer cells by increasing
intracellular free Ca2+. Life Sci. 82:764–771. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carpagnano GE, Foschino-Barbaro MP, Resta
O, Gramiccioni E and Carpagnano F: Endothelin-1 is increased in the
breath condensate of patients with non-small-cell lung cancer.
Oncology. 66:180–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boldrini L, Gisfredi S, Ursino S, Lucchi
M, Melfi F, Mussi A, Basolo F and Fontanini G: Tumour necrosis
factor-alpha: Prognostic role and relationship with interleukin-8
and endothelin-1 in non-small cell lung cancer. Int J Mol Med.
17:887–892. 2006.PubMed/NCBI
|