Introduction
Since Ohashi et al (1) first described intraductal papillary
mucinous neoplasm (IPMN) in 1982, IPMNs have become recognized as
the most common of all cystic tumors of the pancreas, accounting
for up to 70% (2). On the basis of
the location of ductal involvement, IPMNs are divided into three
groups: Main duct IPMN, branch duct IPMN and mixed type IPMN
(3). The first International
Consensus Guidelines for IPMN management were published in 2006
(3) and were later updated in 2012
(4). According to the guidelines,
surgical resection is recommended for all main duct IPMNs due to
the high risk of malignancy (61.6%) and invasive carcinoma (43.1%)
(4,5).
By contrast, the frequency of malignant and invasive IPMNs in
branch duct IPMN were reported to be 25.5 and 17.7%, respectively
(4). The latest International
Consensus Guidelines, however, described worrisome features of
malignancy, including a cyst >3 cm, thickened and enhanced cyst
walls, main pancreatic duct size 5–9 mm, non-enhancing mural
nodule, abrupt change in caliber of duct with distal pancreatic
atrophy and lymphadenopathy (4). No
criterion has been proven accurate in predicting an invasive
progression in main duct IPMN (6).
Several previous studies described predictors of malignancy of main
duct IPMN: Older age, more frequent incidence of jaundice and/or
worsening of diabetes, >15 mm dilatation of the main pancreatic
duct and a mural nodule (5,7). However, 29% of the patients with
malignant main duct IPMN were asymptomatic (5), and those with smaller main duct
dilatation and no mural nodule had invasive carcinomas (7). Previously, a number of additional
predictors of malignancy in branch duct IPMNs were reported:
Elevated tumor markers, an increase of cyst size over time, family
history, multifocal IPMN or obesity (8–12).
An unsettled definition of IPMN malignancy makes
comparison of the described data difficult. Certain reports
included cases with carcinoma in situ into those of
malignant IPMNs, while other studies enrolled patients with
invasive IPMN only into those of malignant disease. The new
International Consensus Guidelines described carcinoma in
situ as high-grade dysplasia (4).
By contrast, the MIB-1 index has been used for
diagnosing malignancy in other diseases. In neuroendocrine tumors,
those with an MIB-1 labeling index of <2% are classified as G1,
and those with an index between 2 and 20% as G2. Tumors with an
index of >20% are classified as neuroendocrine carcinoma
(13). In early breast carcinoma,
patients with a high MIB-1 labeling index have a poor prognosis
(14). As for IPMNs, several reports
have presented data of the MIB-1 labeling index (15–22).
However, confusing criteria for the definition of malignant IPMNs
prevent us from comparing these results.
The aim of the present study was to identify
clinical and pathologic features of invasive IPMN using our cohort
approach that simply classifies patients into two groups:
Non-invasive and invasive IPMN. The present study also aimed to
identify the role of the MIB-1 labeling index as an indicator of
invasive IPMNs.
Materials and methods
Patients
A total of 53 patients with IPMNs who underwent
resection of tumors between 2000 and 2010 were enrolled, in
accordance with the guidelines for informed consent and approval
from the Ethics Committee of our institute. Of these patients, 28
patients exhibited non-invasive IPMN, including three patients with
carcinoma in situ of IPMN, and 25 patients with invasive
IPMN. The neoplasms were classified into non-invasive IPMNs and
invasive IPMNs. Minimally invasive IPMNs were classified into
invasive IPMNs. The neoplasms in the head, neck or uncinate process
of the pancreas were treated with pancreaticoduodenectomy, and
neoplasms in the pancreatic body or tail were treated with open or
laparoscopic distal pancreatectomy accordingly.
Analysis on factors for invasive
IPMN
As for the clinical features in determining
predictive factors for invasive IPMN, age, gender, tumor size, type
of involved duct (main or mixed type vs. branch duct), with or
without symptoms, dilatation of the main duct and a mural nodule in
pre-operative imaging modalities, and the MIB-1 labeling index were
investigated.
Immunohistochemical analysis
The MIB-1 labeling index was assessed by
immunohistochemistry using an avidin-biotin-peroxidase complex
method. Formalin-fixed, paraffin-embedded tissue samples were cut
into 4 µm-thick sections. The sections were deparaffinized in
xylene and rehydrated through a series of decreasing alcohol
concentrations. Following this, they were rinsed three times in
phosphate-buffered saline (PBS), and the sections were immersed in
an absolute methanol solution containing 0.3%
H2O2 for 30 min at room temperature to
inhibit endogenous peroxidase. Antigens were retrieved by
autoclaving sections on slides in 0.01 M (pH 6.0) citrate buffer
for 10 min. After rinsing in PBS, the sections were incubated with
monoclonal mouse anti-human antibody against Ki-67 (Clone, MIB-1;
cat. no. M724001; Dako, Tokyo, Japan; 1:50) overnight at 4°C. A
further wash in PBS was followed by treatment with
peroxidase-labeled anti-mouse antibody (Histofine Simple Stain
Max-PO (M); Nichirei, Tokyo, Japan) as the secondary antibody for
30 min at room temperature. The staining was visualized with
diaminobenzidine. Immunohistochemical evaluations were performed
with a microscope (magnification, ×100). A total of 1,000 tumor
cells were counted to assess positive staining, and the percentages
of positively stained cells were determined as the MIB-1 labeling
index.
Statistical analysis
Categorical variables were evaluated by either the
χ2 or Fisher's exact test. Predictors of invasive IPMNs
were determined with univariate and multivariate analyses using a
logistic regression model. To assess the performance
characteristics of the MIB-1 labeling index, receiver operating
characteristic curves were generated and the area under the curve
was calculated. The survival time was observed between the date of
surgery and date of the last follow-up. Overall survival was
calculated using the Kaplan-Meier method and differences between
the groups were assessed by the log-rank test. The data are
presented as the mean ± standard deviation. All statistical
calculations were performed using SPSS® version 22 (IBM
SPSS, Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
MIB-1 labeling index
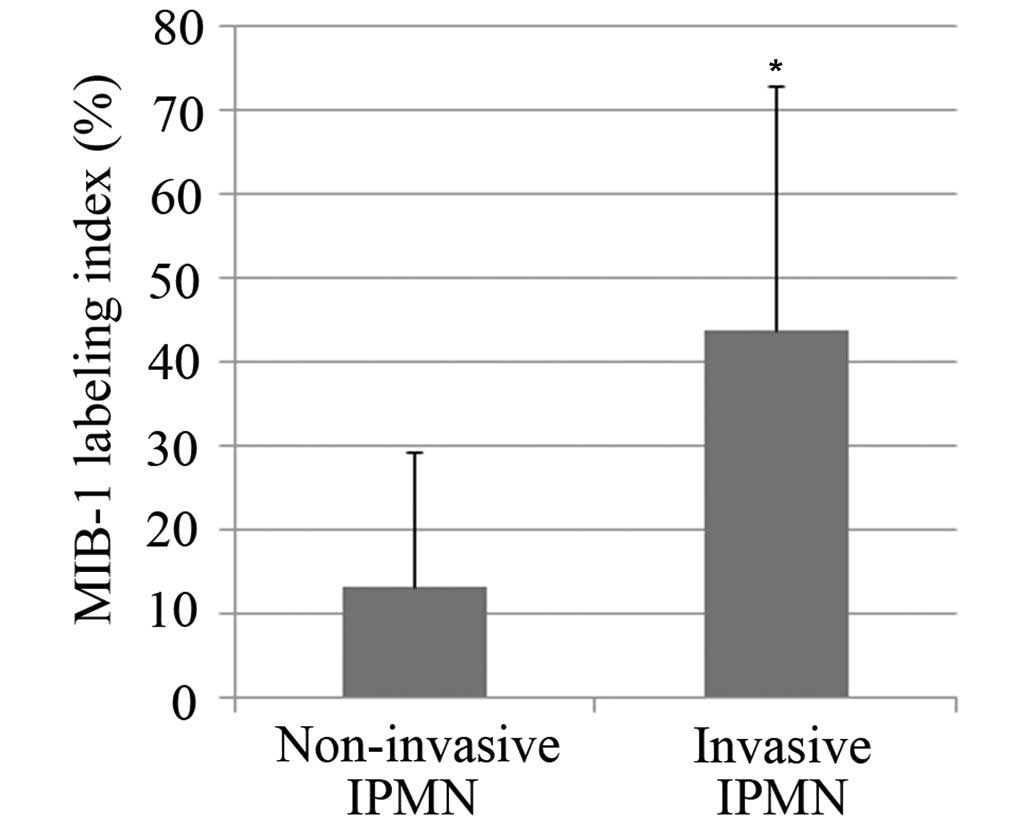
The MIB-1 labeling index was 13.4±15.8 in patients
with non-invasive IPMN and 42.4±30.3 in patients with invasive IPMN
(Fig. 1). A statistically significant
difference was observed between the groups (P<0.001). A
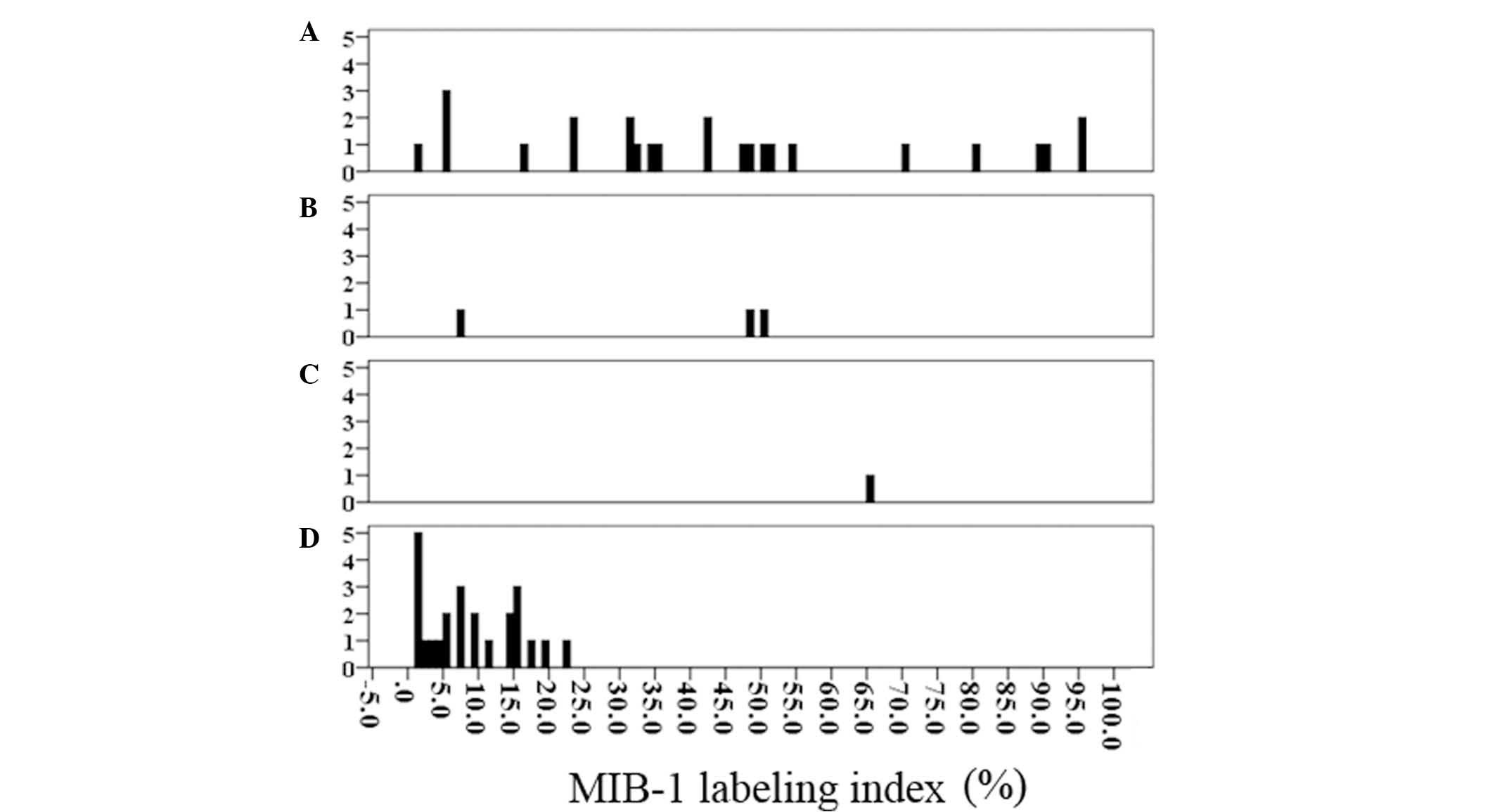
histogram of the MIB labeling index was generated according to the
pathological grade (Fig. 2). The
labeling index of four patients with invasive IPMN was under 5% (1
patient, 1% and 3 patients, 5%), while that of three patients with
non-invasive IPMN (2 with carcinoma in situ component and 1
with high grade dysplasia) was over the mean labeling index of
patients with invasive IPMN.
Performance of MIB-1 labeling
index
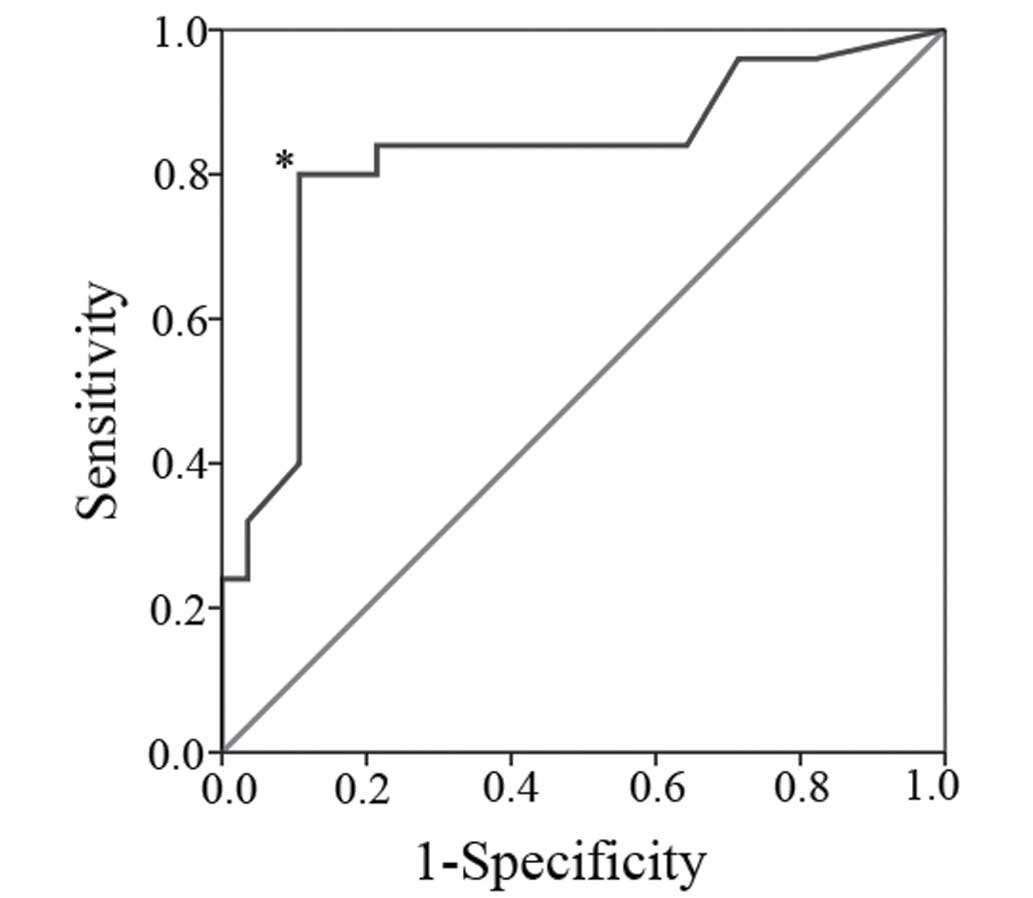
The receiver operating characteristic curve is shown
in Fig. 3. The calculated area under
the curve was 0.822. At a cut-off level set to an index of 15.5%,
sensitivity was 0.84 and specificity was 0.79. This resulted in an
accuracy of 81% and four patients with invasive IPMN would be
misdiagnosed. As shown in Fig. 2, the
four patients with invasive IPMN exhibited 1, 5, 5 and 5% in the
MIB labeling index. Therefore, it was estimated that the cut-off
level of the MIB-1 labeling index be set to 15.5% as a lower
cut-off level was more likely to be inaccurate.
Comparison of characteristics of
patients between non-invasive and invasive IPMN
Table I shows the
comparison of characteristics of patients between non-invasive and
invasive IPMN. The mean ages of patients with non-invasive IPMNs
and invasive IPMNs were 66.0±8.7 and 69.4±9.9, respectively. As for
the MIB-1 labeling index, patients with ≥15.5% were classified into
the higher group and those with <15.5% into the lower group. The
existence of a mural nodule and a higher MIB-1 labeling index were
significantly more frequent in the patients with invasive IPMN
compared with those with non-invasive IPMN (P=0.011 and P<0.001,
respectively). No statistically significant difference was observed
between non-invasive and invasive IPMN in the other examined
characteristics.
 | Table I.Comparison of various characteristics
between patients with non-invasive and invasive intraductal
papillary mucinous neoplasm. |
Table I.
Comparison of various characteristics
between patients with non-invasive and invasive intraductal
papillary mucinous neoplasm.
| Characteristic | Non-invasive
(n=28) | Invasive (n=25) | P-value |
|---|
| Age (mean ± SD) | 66.0±8.7 | 69.4±9.9 | 0.190 |
| Gender |
|
| 0.184 |
| Male | 14 | 17 |
|
|
Female | 14 | 8 |
|
| Involved duct |
|
| 0.059 |
|
Branch | 10 | 3 |
|
| Main or
Mixed | 18 | 22 |
|
| Size |
|
| 0.743 |
|
<3.0 | 19 | 18 |
|
| ≥3.0 | 9 | 7 |
|
| Symptom |
|
| 0.694 |
| No | 25 | 21 |
|
| Yes | 3 | 4 |
|
| Main Duct
Dilatation |
|
| 0.509 |
| No | 7 | 4 |
|
| Yes | 21 | 21 |
|
| Mural Nodule |
|
| 0.011 |
| No | 25 | 14 |
|
| Yes | 3 | 11 |
|
| MIB-1 labeling
index |
|
| <0.001 |
|
<15.5% | 25 | 4 |
|
|
≥15.5% | 3 | 21 |
|
Univariate and multivariate analyses
of patients with non-invasive or invasive IPMN
To determine which factors are predictors of
invasive IPMN, each one was measured using a logistic regression
model. The results are shown in Table
II. In the univariate analysis, the existence of a mural nodule
and the MIB-1 labeling index achieved statistically significant
differences (P=0.01 and P<0.001, respectively). In the
multivariate analysis, the existence of a mural nodule (hazard
ratio, 6.187; 95% confidential interval, 1.039–36.861; P=0.045) and
the MIB-1 labeling (hazard ratio, 18.692; 95% confidential
interval, 4.171–83.760; P<0.001) were independent predictors of
invasive IPMN.
 | Table II.Univariate and multivariate analysis
of potential predictive factors for invasive invasive intraductal
papillary mucinous neoplasm. |
Table II.
Univariate and multivariate analysis
of potential predictive factors for invasive invasive intraductal
papillary mucinous neoplasm.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Tumor size (>3
cm) | 0.821 | 0.252–2.670 | 0.743 |
|
|
|
| Main duct or mixed
type | 4.074 | 0.972–17.071 | 0.055 |
|
|
|
| Symptoms | 1.587 | 0.319–7.905 | 0.573 |
|
|
|
| Main duct
dilatation | 1.750 | 0.445–6.882 | 0.423 |
|
|
|
| Mural nodule | 6.548 | 1.560–27.484 | 0.010 | 6.187 | 1.039–36.861 | 0.045 |
| MIB-1 labeling
index (≥15.5%) | 19.250 | 4.750–78.011 |
<0.001 | 18.692 | 4.171–83.760 |
<0.001 |
Survival of patients
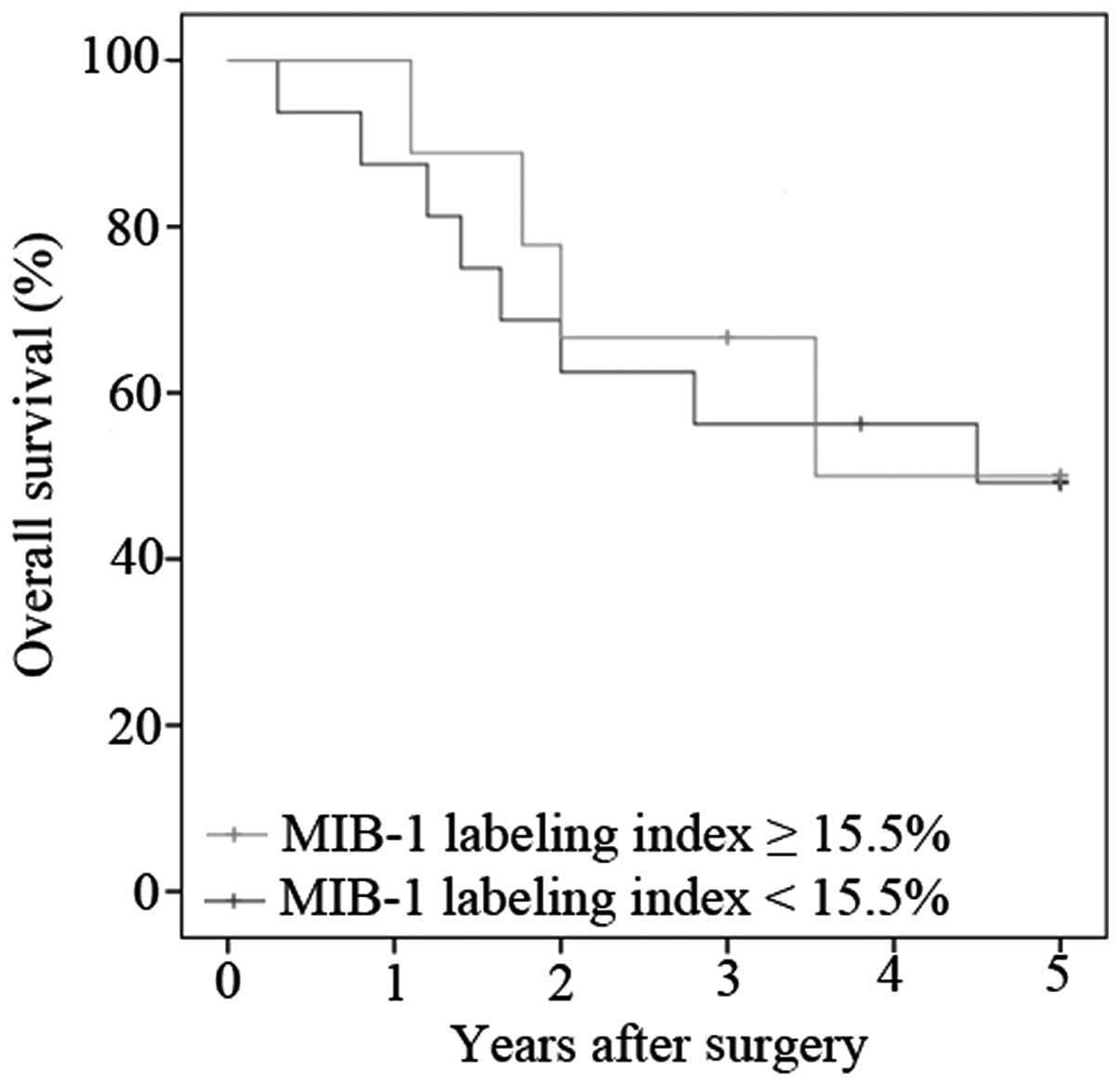
The MIB-1 labeling index of the patients with
invasive IPMN was 43.8±29.1. The present study decided to classify
the patients into two groups to evaluate prognosis of those with
invasive IPMN: Patients with a labeling index ≥50% and the patients
with a labeling index <50%. Fig. 4
shows the overall survival of the patients with invasive IPMN
according to the level of the labeling index. The median survival
of patients with a lower MIB-1 labeling index was 4.50 years,
whereas that of those with a higher index was 3.53 years. No
statistically significant differences were observed between the
groups (P=0.798).
Discussion
The present results revealed that the MIB-1 labeling
index and the existence of a mural nodule were predictive factors
for invasive IPMN. Takeshita et al (22) reported the MIB-1 labeling index as a
prognostic factor; however, to the best of our knowledge, this is
the first report describing the MIB-1 labeling index as a predictor
of invasive IPMNs. The report by Takeshita et al (22) revealed statistically significant
differences of the MIB-1 labeling index between low- or
intermediate-grade dysplasia and high-grade dysplasia, or carcinoma
in situ component, or an invasive component of IPMN
(22). Other previous reports have
described the MIB-1 labeling index in the context of cell
proliferation (15–21). Abe et al (18) reported that the MIB-1 labeling index
increased in accordance with adenoma, borderline lesion and
carcinoma in situ. However, as a result of the confusing
criteria for defining malignant IPMN, it is difficult to compare
these results (18). Therefore, the
present study classified the patients into two groups:
Non-invasive, including IPMNs with carcinoma in situ
component, and invasive IPMN. By contrast, the existence of a mural
nodule has been reported to be a predictive factor for invasive
IPMN (6,7).
As for the performance of the MIB-1 index as a
predictor of invasive IPMNs, the receiver operating characteristic
curve proved that an index threshold of 15.5% was the best to
distinguish between non-invasive and invasive IPMN. However, 4/25
patients with invasive IPMN exhibited 1, 5, 5 and 5% in the MIB
labeling index. These patients could therefore not be detected by a
lowered cut-off level. Among these patients, three patients
exhibited a cyst size >3.0 cm, main duct dilation, or an
abnormal tumor marker. Additionally, two of those exhibtied a mural
nodule. They could be detected as high risk for malignancy by the
worrisome features and/or high risk stigmata. Therefore, a
combination of clinical features, including worrisome features and
high risk stigmata with the MIB-1 labeling index would be
useful.
In terms of the prognosis of invasive IPMNs, the
analysis of the present study revealed no statistical significance
between patients with a lower or higher MIB-1 labeling index.
Takeshita et al (22) reported
that IPMN with low- or intermediate-grade dysplasia exhibited a
significantly improved prognosis compared with IPMN with an
associated invasive carcinoma, while no statistically significant
difference was observed between the prognosis of IPMN with high
grade dysplasia and with an associated invasive carcinoma (22). It was also reported that the MIB-1
labeling index significantly increased from 1.8% in IPMN with low-
or intermediate-grade dysplasia to 14–23% in carcinoma, concluding
that a sudden change in proliferative activity occurred between the
two. A difference in classification of IPMN existed between the
previous report and the present study, which classified high grade
dysplasia into non-invasive IPMN.
To use the MIB-1 labeling index as a predictor,
pre-operative assessment is required. Fine-needle aspiration biopsy
using endoscopic ultrasonography can provide a preoperative
opportunity to assess the labeling index. However, due to concerns
over intra-abdominal dissemination, fine-needle aspiration is not
always performed prior to operation (23,24). The
feasibility of assessing the MIB-1 labeling index using fine needle
aspiration biopsy under endoscopic ultrasonography must be
determined by a clinical research in the future since tumors have
heterogeneity in the MIB-1 labeling index.
In conclusion, the MIB-1 labeling index and the
existence of mural nodules were proven to be useful as an indicator
of invasive IPMN. Although malignancy of certain patients failed to
be detected by the MIB-1 labeling index, a combination of worrisome
features and high risk stigmata, including the existence of mural
nodule) may assist in accurately diagnosing patients with invasive
IPMN. The MIB-1 index must be considered as a candidate for the
classification of IPMNs.
Glossary
Abbreviations
Abbreviations:
|
IPMN
|
intraductal papillary mucinous
neoplasm
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Ohashi K, Murakami Y, Maruyama M,
Takekoshi T, Ohta H, Ohhashi I, Takagi K and Kato K: Four cases of
mucous secreting pancreatic cancer. Prog Dig Endosc. 20:348–351.
1982.
|
|
2
|
Werner J, Fritz S and Büchler MW:
Intraductal papillary mucinous neoplasms of the pancreas-a surgical
disease. Nat Rev Gastroenterol Hepatol. 9:253–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanaka M, Chari S, Adsay V, Fernandez-del
Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K and Matsuno
S: International Association of Pancreatology: International
consensus guidelines for management of intraductal papillary
mucinous neoplasms and mucinous cystic neoplasms of the pancreas.
Pancreatology. 6:17–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanaka M, del Fernández Castillo C, Adsay
V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB,
Schmidt CM, et al: International consensus guidelines 2012 for the
management of IPMN and MCN of the pancreas. Pancreatology.
12:183–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salvia R, Fernández-del Castillo C, Bassi
C, Thayer SP, Falconi M, Mantovani W, Pederzoli P and Warshaw AL:
Main-duct intraductal papillary mucinous neoplasms of the pancreas:
Clinical predictors of malignancy and long-term survival following
resection. Ann Surg. 239:678–685; discussion 685–687. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roch AM, Ceppa EP, Al-Haddad MA, DeWitt
JM, House MG, Zyromski NJ, Nakeeb A and Schmidt CM: The natural
history of main duct-involved, mixed-type intraductal papillary
mucinous neoplasms: Parameters predictive of progression. Ann Surg.
260:680–688; discussion 688–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugiyama M, Izumisato Y, Abe N, Masaki T,
Mori T and Atomi Y: Predictive factors for malignancy in
intraductal papillary-mucinous tumors of the pancreas. Br J Surg.
90:1244–1249. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fritz S, Hackert T, Hinz U, Hartwig W,
Büchler MW and Werner J: Role of serum carbohydrate antigen 19-9
and carcinoembryonic antigen in distinguishing between benign and
invasive intraductal papillary mucinous neoplasm of the pancreas.
Br J Surg. 98:104–110. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang MJ, Jang JY, Kim SJ, Lee KB, Ryu JK,
Kim YT, Yoon YB and Kim SW: Cyst growth rate predicts malignancy in
patients with branch duct intraductal papillary mucinous neoplasms.
Clin Gastroenterol Hepatol. 9:87–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He J, Cameron JL, Ahuja N, Makary MA,
Hirose K, Choti MA, Schulick RD, Hruban RH, Pawlik TM and Wolfgang
CL: Is it necessary to follow patients after resection of a benign
pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg.
216:657–665; discussion 665–667. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fritz S, Schirren M, Klauss M, Bergmann F,
Hackert T, Hartwig W, Strobel O, Grenacher L, Büchler MW and Werner
J: Clinicopathologic characteristics of patients with resected
multifocal intraductal papillary mucinous neoplasm of the pancreas.
Surgery. 152(3 Suppl 1): S74–S80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sturm EC, Roch AM, Shaffer KM, Schmidt CM
II, Lee SJ, Zyromski NJ, Pitt HA, Dewitt JM, Al-Haddad MA, Waters
JA and Schmidt CM: Obesity increases malignant risk in patients
with branch-duct intraductal papillary mucinous neoplasm. Surgery.
154:803–808; discussion 808–809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang LH, Gonen M, Hedvat C, Modlin IM and
Klimstra DS: Objective quantification of the Ki67 proliferative
index in neuroendocrine tumors of the gastroenteropancreatic
system: A comparison of digital image analysis with manual methods.
Am J Surg Pathol. 36:1761–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Viale G, Giobbie-Hurder A, Regan MM,
Coates AS, Mastropasqua MG, Dell'Orto P, Maiorano E, MacGrogan G,
Braye SG, Ohlschlegel C, et al: Prognostic and predictive value of
centrally reviewed Ki-67 labeling index in postmenopausal women
with endocrine-responsive breast cancer: Results from Breast
International Group Trial 1–98 comparing adjuvant tamoxifen with
letrozole. J Clin Oncol. 26:5569–5575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Semba S, Moriya T, Kimura W and Yamakawa
M: Phosphrylated Akt/PKB controls cell growth and apoptosis in
intraductal papillary-mucinous tumor and invasive ductal
adenocarcinoma of the pancreas. Pancreas. 26:250–257. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kataoka TR, Ioka T, Tsukamoto Y, Matsumura
M, Ishiguro S and Nishizawa Y: Nuclear expression of STAT5 in
intraductal papillary mucinous neoplasms of the pancreas. Int J
Surg Pathol. 15:277–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okada K, Masuda N, Fukai Y, Shimura T,
Nishida Y, Hosouchi Y, Kashiwabara K, Nakajima T and Kuwano H:
Immunohistochemical expression of 14-3-3 sigma protein in
intraductal papillary mucinous tumor and invasive ductal carcinoma
of the pancreas. Anticancer Res. 26:3105–3110. 2006.PubMed/NCBI
|
|
18
|
Abe K, Suda K, Arakawa A, Yamasaki S,
Sonoue H, Mitani K and Nobukawa B: Different patterns of p16INK4A
and p53 protein expressions in intraductal papillary-mucinous
neoplasms and pancreatic intraepithelial neoplasia. Pancreas.
34:85–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okimura A, Hirano H, Nishigami T, Ueyama
S, Tachibana S, Fukuda Y, Yamanegi K, Ohyama H, Terada N and
Nakasho K: Immunohistochemical analysis of E cadherin,
beta-catenin, CD44s, and CD44v6 expressions, and Ki-67 labeling
index in intraductal papillary mucinous neoplasms of the pancreas
and associated invasive carcinomas. Med Mol Morphol. 42:222–229.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Islam HK, Fujioka Y, Tomidokoro T, Sugiura
H, Takahashi T, Kondo S and Katoh H: Immunohistochemical analysis
of expression of molecular biologic factors in intraductal
papillary-mucinous tumors of pancreas-diagnostic and biologic
significance. Hepatogastroenterology. 46:2599–2605. 1999.PubMed/NCBI
|
|
21
|
Terada T, Ohta T, Kitamura Y, Ashida K and
Matsunaga Y: Cell proliferative activity in intraductal
papillary-mucinous neoplasms and invasive ductal adenocarcinomas of
the pancreas: An immunohistochemical study. Arch Pathol Lab Med.
122:42–46. 1998.PubMed/NCBI
|
|
22
|
Takeshita A, Kimura W, Hirai I, Takasu N,
Moriya T, Tezuka K and Watanabe T: Clinicopathologic study of the
MIB-1 labeling index (Ki67) and postoperative prognosis for
intraductal papillary mucinous neoplasms and ordinary ductal
adenocarcinoma. Pancreas. 41:114–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamao K, Yanagisawa A, Takahashi K, Kimura
W, Doi R, Fukushima N, Ohike N, Shimizu M, Hatori T, Nobukawa B, et
al: Clinicopathological features and prognosis of mucinous cystic
neoplasm with ovarian-type stroma: A multi-institutional study of
the Japan pancreas society. Pancreas. 40:67–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirooka Y, Goto H, Itoh A, Hashimoto S,
Niwa K, Ishikawa H, Okada N, Itoh T and Kawashima H: Case of
intraductal papillary mucinous tumor in which endosonography-guided
fine-needle aspiration biopsy caused dissemination. J Gastroenterol
Hepatol. 18:1323–1324. 2003. View Article : Google Scholar : PubMed/NCBI
|