Introduction
The solitary pulmonary nodule (SPN) is defined as an
isolated, single lesion of round or oval shape, with a diameter of
≤3 cm, which is located within the lung parenchyma, surrounded
entirely by gas-containing lung tissue. Such lesions are not
accompanied by lung atelectasis, hilar enlargement or pleural
effusion (1,2). With the established role of computed
tomography (CT) screening for lung cancer and the wide application
of high-resolution CT, SPNs are detected at an increasing rate
(3–5).
SPN management basically comprises the implementation of immediate
surgical treatment (6,7). Over the last decade, video-assisted
thoracoscopic surgery (VATS) has become a useful tool in the
diagnosis and treatment of SPNs (8,9). However,
due to the small size of the nodules, preoperative localization is
crucial for the success of VATS.
The preoperative localization methods include
microvascular embolisation coils, hookwire insertion and injection
of dye (10–15). Each method has its merits and
drawbacks. We developed a new technique using a combination of
methylene blue and an ultrathin bronchoscope under radial probe
endobronchial ultrasound (RP-EBUS) guidance. this technique was
applied in 48 patients and achieved effective, safe and convenient
SPN localization.
Materials and methods
Patients
Between January, 2013 and September, 2014,
RP-EBUS-guided SPN localization with an ultrathin bronchoscope and
methylene blue, followed by VATS, was conducted on 48 SPNs from 48
patients who underwent CT examination at the Nanjing Clinical
Center of Respiratory Diseases and Imaging (Nanjing, China). The
study included 18 men and 30 women, with a mean age of 54 years
(range, 41–72 years). Of the 48 patients, 12 had a cancer history.
The patient characteristics are summarized in Table I. The study protocol was approved by
the Ethics Committee of the Nanjing Chest Hospital and all the
patients provided written informed consent.
 | Table I.Patient characteristics (n=48). |
Table I.
Patient characteristics (n=48).
| Characteristics | No. of patients
(%) |
|---|
| Age, years |
|
| ≥60 | 20 (41.7) |
|
<60 | 28 (58.3) |
| Gender |
|
| Male | 18 (37.5) |
|
Female | 30 (62.5) |
| Cancer history |
|
| Yes | 12 (25.0) |
| No | 36 (75.0) |
| Smoking history |
|
|
Smoker | 15 (31.3) |
|
Non-smoker | 33 (68.7) |
RP-EBUS-guided combination of
ultrafine bronchoscopy and methylene blue localization
Each patient had been diagnosed with a pulmonary
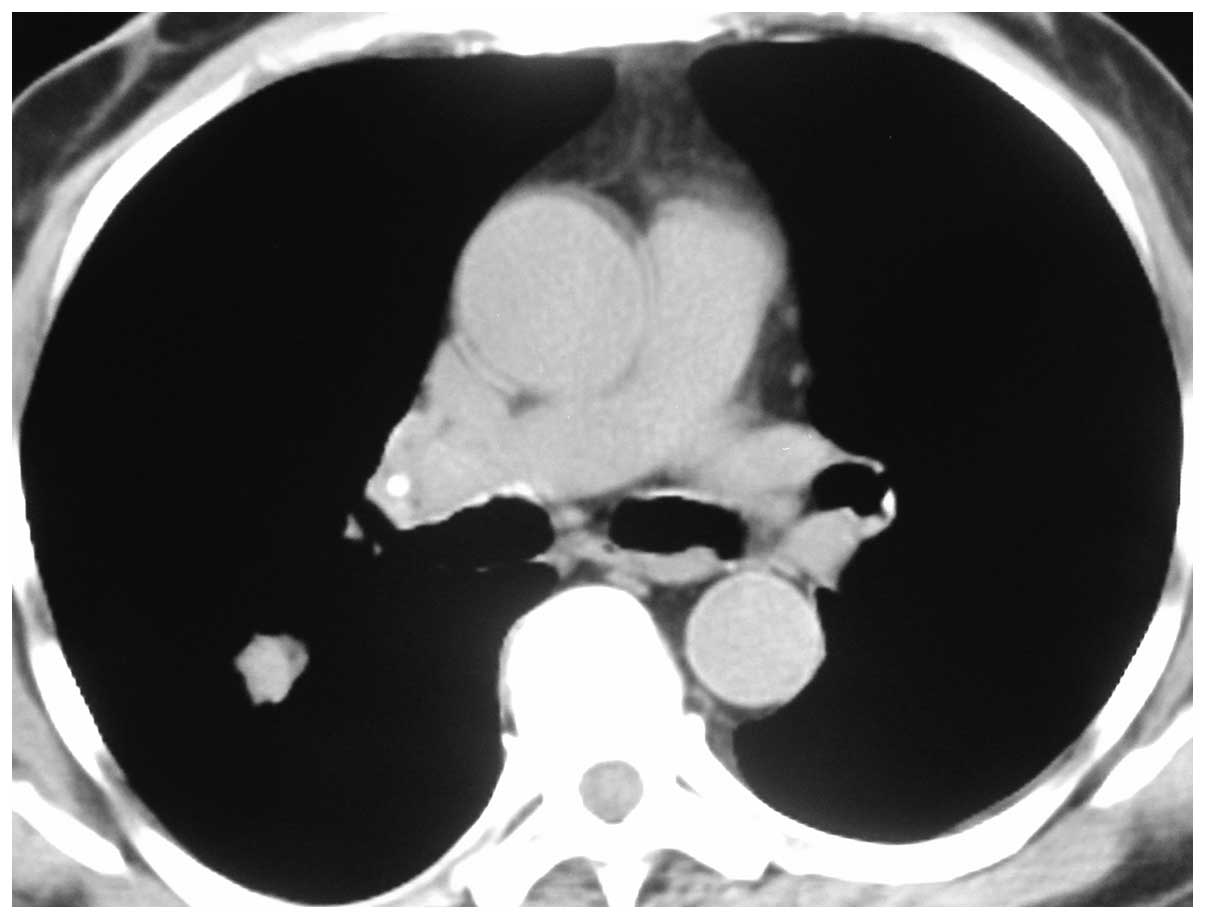
nodule on a CT scan of the chest, measuring 10 mm (Fig. 1). RP-EBUS scanning was conducted under
anesthesia, with percutaneous blood oxygen saturation monitoring
and continuous electrocardiography during the examination process.
A 2.0-mm, 20-MHz radial mechanical transducer type endobronchial
ultrasonic probe (UM-BS20-26R; Olympus, Tokyo, Japan) with a
flexible balloon sheath (MAJ-643R; Olympus) was introduced through
the 2.8-mm channel of a flexible bronchoscope (XBF-22L; Olympus).
The probe was connected to the endobronchial ultrasonography unit
(EU-M 20/30; Olympus). The visible bronchial segment was examined
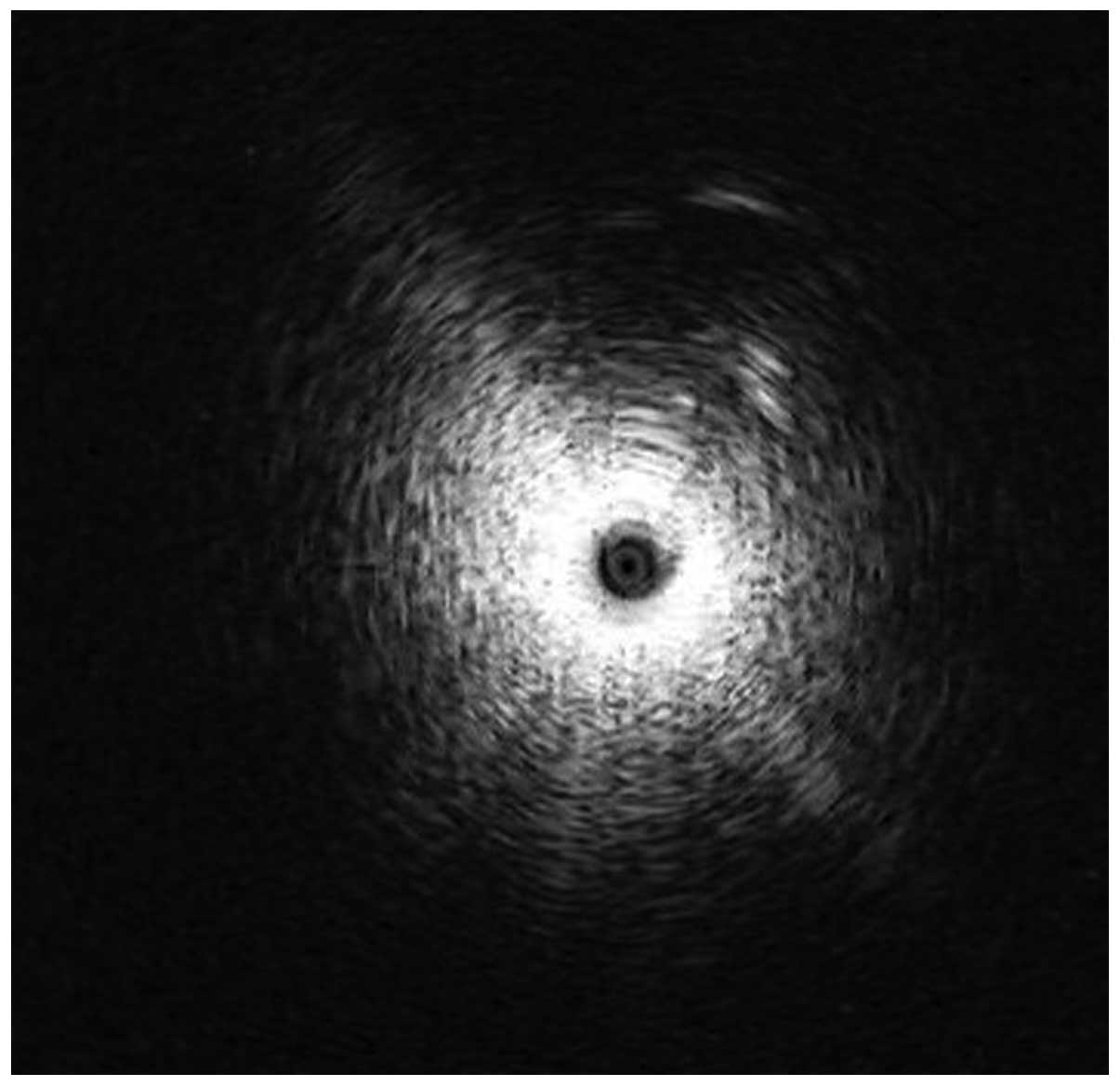
until the characteristic ultrasound signal indicating the presence
of a solid lesion (Fig. 2). The EBUS
probe was removed and 0.3 ml methylene blue was injected at the
marked point, according to the angle and depth data.
VATS
Thoracoscopic segmentectomy was performed under
general anesthesia using single-lung ventilation. The technique
utilized three incisions. The observation port was ~1 cm in length
at the midaxillary line in the 6th or 7th intercostal space. The
main operation port was placed in the 3rd or 4th intercostal space
at the anterior axillary line. The accessory operation port was
usually placed at the posterior axillary line in the 6th
intercostal space. Both operation ports were ~2 cm in length.
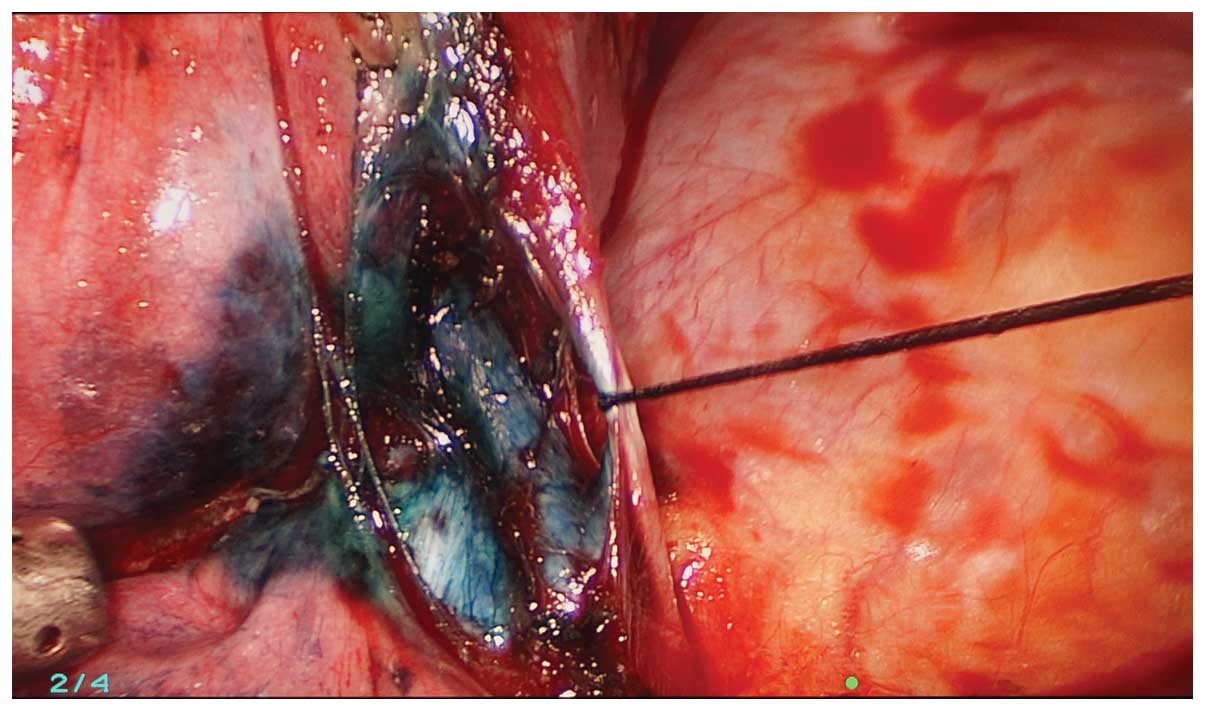
The methylene blue was visualized during the
exploration and the location of the lesion was confirmed (Fig. 3). The initial step was dissection of
the hilar lymph nodes to ensure safe sublobar resection. The lymph
nodes were sent for frozen section analysis and sublobar resection
was abandoned if positive nodes were identified.
The approach to thoracoscopic segmentectomy begun
with ligation of the segmental pulmonary vein and artery using
thoracoscopic linear mechanical staplers. The bronchus was further
isolated and dissected by stapler. Subsequently, the ipsilateral
lung was temporarily reinflated to help identify the segmental
fissures. After the fissures were marked with electrocautery and
squeezed by long kelly clamps, the segmental plane was finished
with an endostapler. In certain cases, we had to convert to a
thoracotomy due to problems such as adhesions or failure of
localization.
Results
Characteristics of the SPNs
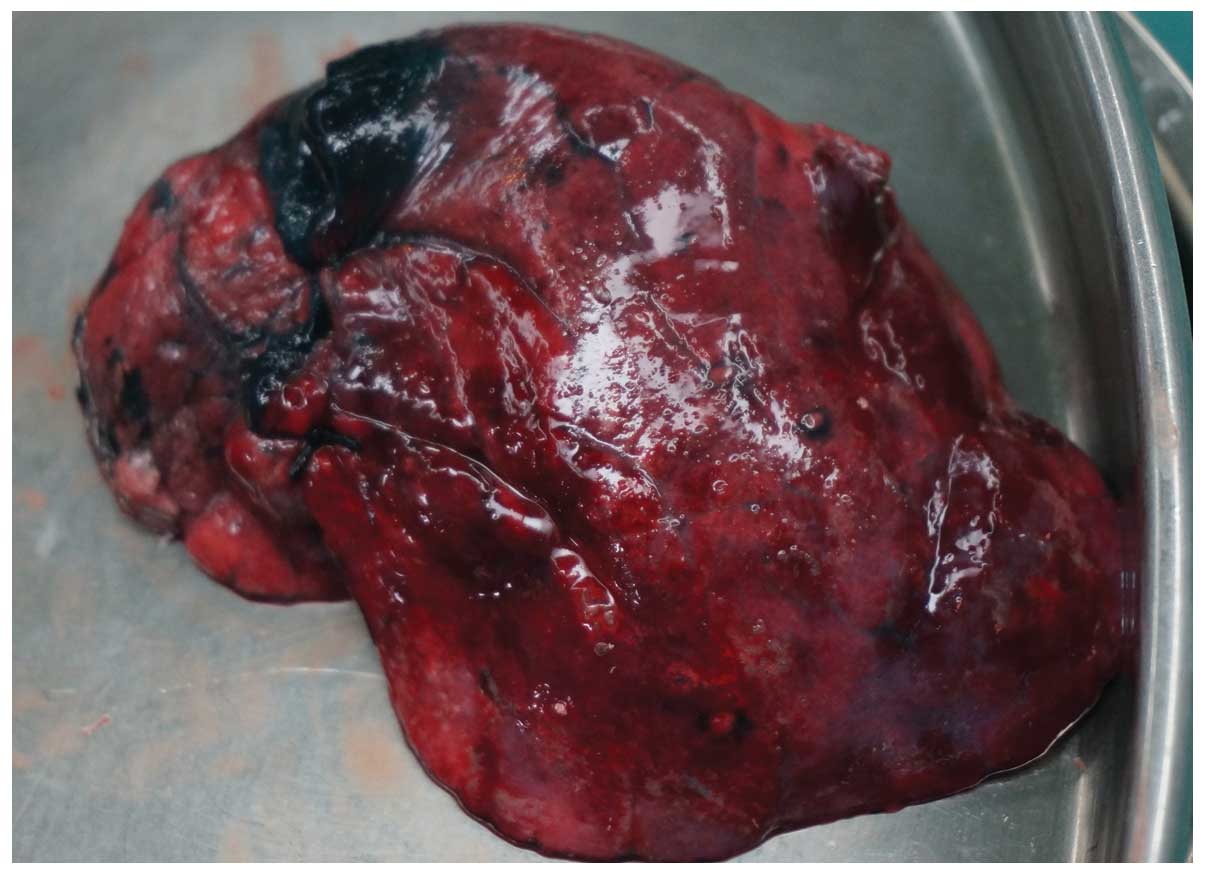
In 35 (72.9%) of the 48 patients, the methylene blue
was visualized in or adjacent to the pulmonary nodule as documented
following VATS resection (Fig. 4).
The maximal diameter of the SPNs, their distance from the pleural
surface and their histological diagnosis are summarized in Tables II and III. The diameter of the nodules ± standard
deviation (SD) was 12.8±4.2 mm and located at a distance of
11.2±9.7 mm from the pleural surface. A total of 35 nodules were
malignant and 13 were benign. A total of 12 lesions were located in
the right upper lobe, 10 were in the right lower lobe, 16 in the
left upper lobe and 10 in the left lower lobe.
 | Table II.Characteristics of the SPNs
(n=48). |
Table II.
Characteristics of the SPNs
(n=48).
| Characteristics | Value |
|---|
| Diameter, mm | 12.8±4.2 |
| Distance from pleural
surface, mm | 11.2±9.7 |
| Location, no.
(%) |
|
| Upper
lobe of right lung | 12 (25.0) |
| Lower
lobe of right lung | 10 (20.8) |
| Upper
lobe of left lung | 16 (33.3) |
| Lower
lobe of left lung | 10 (20.8) |
| Density, no. (%) |
|
| GGN | 18 (37.5) |
| Solid
nodule | 30 (62.5) |
 | Table III.Histological diagnosis of SPNs. |
Table III.
Histological diagnosis of SPNs.
| Types | No. of patients
(%) |
|---|
| Benign | 13 (27.1) |
|
Hamartoma | 4 (8.3) |
| Pulmonary
tuberculosis | 3 (6.3) |
|
Infammatory pseudotumor | 6 (12.5) |
| Malignant | 35 (72.9) |
| Atypical
adenomatous hyperplasia | 4 (8.3) |
|
Adenocarcinoma in
situ | 5 (10.4) |
| Minimally
invasive adenocarcinoma | 7 (14.6) |
|
Adenocarcinoma | 18 (37.5) |
| Squamous
cell carcinoma | 1 (2.1) |
RP-EBUS-guided SPN localization
The duration of localization, measured from the
administration of local anesthesia, ranged between 10 and 28 min
(mean, 15 min) and the number of ultrafine bronchoscope insertions
or adjustments ranged between 3 and 6 (mean, 3). The only
complication of RP-EBUS-guided localization with the combination of
an ultrafine bronchoscope and methylene blue was asymptomatic
hemorrhage, which was observed in 8.3% of the patients. VATS was
performed in all 48 patients; 38 cases underwent wedge resection,
including 13 benign and 25 malignant lesions and the remaining 8
patients underwent lobectomy and lymphadenectomy. The mean surgery
duration ± SD for wedge resection and lobectomy was 25±8 and 90±30
min, respectively.
In two cases the procedure was converted to
thoracotomy: One SPN strongly adhered to the pleural surface and
the other was difficult to localize. The characteristics of the
RP-EBUS-guided localization with an ultrafine bronchoscope and
methylene blue and subsequent VATS are summarized in Table IV.
 | Table IV.Characteristics of RP-EBUS-guided SPN
localization with a combination of an ultrafine bronchoscope and
methylene blue. |
Table IV.
Characteristics of RP-EBUS-guided SPN
localization with a combination of an ultrafine bronchoscope and
methylene blue.
| Characteristics | Values |
|---|
| Complications, no.
(%) |
|
|
Pneumothorax | 0 (0.0) |
|
Hemorrhage | 4 (8.3) |
|
Pneumothorax and
hemorrhage | 0 (0.0) |
| Duration, min |
|
|
Range | 10–28 |
| Mean | 15 |
| Ultrafine
bronchoscope insertions, no. |
|
|
Range | 3–6 |
| Mean | 3 |
Discussion
VATS is a useful minimally invasive procedure for
the diagnosis and treatment of peripheral small pulmonary nodules.
However, as such lesions may not be visible or palpable during
VATS, a conversion from VATS to thoracotomy is occasionally
conducted following failure to localize these lesions (16,17).
Therefore, it is crucial to accurately localize lesions prior to
VATS, particularly in the case of small or faint nodules.
The most common localization method is the CT-guided
insertion of a hookwire. However, this technique is associated with
the development of pneumothorax and wires may be dislodged with
movement (18). The most commonly
used dye, methylene blue, may diffuse quickly to the uninvolved
pleural surface and make localization difficult (19).
In the present study, 35 lesions were successfully
localized. The mean duration of localization ± SD was 15.7±8.3 min
and the mean surgery duration for wedge resection and lobectomy was
25±8 and 90±30 min, respectively. All 48 lesions were
pathologically examined. Malignant lesions accounted for 72.9% of
the cases; this result was similar to previous reports (20–22). We
suggest that this may be due to the benign diagnoses of certain
patients in this study, who were selected based on imaging results
rather than pathological diagnosis.
Although the RP-EBUS-guided method appears to be
inferior to the CT-guided hookwire system in terms of successful
localization, our technique provides several distinct advantages
over other reported techniques, such as introduction of the probe
through a natural lumen, absence of pneumothorax, low bleeding
rate, no air embolism and no radiation damage.
Our study had several limitations, including the
small number of patients and the fact that our localization method
was not compared to other methods. Further comparative studies are
required to provide a definitive answer on the optimal localization
method prior to VATS.
In conclusion, RP-EBUS-guided combination of
ultrafine bronchoscopy and methylene blue for the localization of
SPNs prior to VATS is an effective, safe and convenient
localization method.
Acknowledgements
The present study was supported in part by a grant
from ‘Twelve-Five Plan’, the major program of Nanjing Medical
Science and Technique Development Foundation (Molecular Mechanism
Study on Metastasis and Clinical Efficacy Prediction of Non-small
Cell Lung Cancer) to Li-Ke Yu.
References
|
1
|
Ost D, Fein AM and Feinsilver SH: Clinical
practice. The solitary pulmonary nodule. N Engl J Med.
348:2535–2542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu C, Hao K, Song Y, et al: Early
diagnosis of solitary pulmonary nodules. J Thorac Dis. 5:830–840.
2013.PubMed/NCBI
|
|
3
|
Aberle DR, DeMello S, Berg CD, et al:
National Lung Screening Trial Research Team: Results of the two
incidence screenings in the National Lung Screening Trial. N Engl J
Med. 369:920–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McWilliams A, Tammemagi MC, Mayo JR, et
al: Probability of cancer in pulmonary nodules detected on first
screening CT. N Engl J Med. 369:910–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel VK, Naik SK, Naidich DP, et al: A
practical algorithmic approach to the diagnosis and management of
solitary pulmonary nodules: part 1: radiologic characteristics and
imaging modalities. Chest. 143:825–839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cummings SR, Lillington GA and Richard RJ:
Estimating the probability of malignancy in solitary pulmonary
nodules. A Bayesian approach. Am Rev Respir Dis. 134:449–452.
1986.PubMed/NCBI
|
|
7
|
Mery CM, Pappas AN, Bueno R, et al:
Relationship between a history of antecedent cancer and the
probability of malignancy for a solitary pulmonary nodule. Chest.
125:2175–2181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao H, Pu Q, Mei J, et al: Value of
video-assisted thoracic surgery core needle biopsy in the selection
of surgical approaches for indeterminate pulmonary nodules. Ann
Thorac Surg. 95:7722013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernard A: Resection of pulmonary nodules
using video-assisted thoracic surgery. The Thorax Group. Ann Thorac
Surg. 61:202–204. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Powell TI, Jangra D, Clifton JC, et al:
Peripheral lung nodules: fluoroscopically guided video-assisted
thoracoscopic resection after computed tomography-guided
localization using platinum microcoils. Ann Surg. 240:481–488.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito H, Minamiya Y, Matsuzaki I, et al:
Indication for preoperative localization of small peripheral
pulmonary nodules in thoracoscopic surgery. J Thorac Cardiovasc
Surg. 124:1198–1202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen W, Chen L, Yang S, et al: A novel
technique for localization of small pulmonary nodules. Chest.
131:1526–1531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kastl S, Langwieler TE, Krupski-Berdien G,
et al: Percutaneous localization of pulmonary nodules prior to
thoracoscopic surgery by CT-guided hook-wire. Anticancer Res.
26:3123–3126. 2006.PubMed/NCBI
|
|
14
|
Partik BL, Leung AN, Müller MR, et al:
Using a dedicated lung-marker system for localization of pulmonary
nodules before thoracoscopic surgery. AJR Am J Roentgenol.
180:805–809. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chella A, Lucchi M, Ambrogi MC, et al: A
pilot study of the role of TC-99 radionuclide in localization of
pulmonary nodular lesions for thoracoscopic resection. Eur J
Cardiothorac Surg. 18:17–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Zhou J, Zhang J, et al:
Video-assisted thoracoscopic solitary pulmonary nodule resection
after CT-guided hookwire localization: 43 cases report and
literature review. Surg Endosc. 25:1723–1729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki K, Nagai K, Yoshida J, et al:
Video-assisted thoracoscopic surgery for small indeterminate
pulmonary nodules: indications for preoperative marking. Chest.
115:563–568. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horan TA, Pinheiro PM, Araújo LM, et al:
Massive gas embolism during pulmonary nodule hook wire
localization. Ann Thorac Surg. 73:1647–1649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwasaki Y, Nagata K, Yuba T, et al:
Fluoroscopy-guided barium marking for localizing small pulmonary
lesions before video-assisted thoracic surgery. Respir Med.
99:285–289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pittet O, Christodoulou M, Pezzetta E, et
al: Video-assisted thoracoscopic resection of a small pulmonary
nodule after computed tomography-guided localization with a
hook-wire system. Experience in 45 consecutive patients. World J
Surg. 31:575–578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ciriaco P, Negri G, Puglisi A, et al:
Video-assisted thoracoscopic surgery for pulmonary nodules:
rationale for preoperative computed tomography-guided hookwire
localization. Eur J Cardiothorac Surg. 25:429–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirai S, Hamanaka Y, Mitsui N, et al: Role
of video-assisted thoracic surgery for the diagnosis of
indeterminate pulmonary nodule. Ann Thorac Cardiovasc Surg.
12:388–392. 2006.PubMed/NCBI
|