Introduction
Irinotecan (CPT-11), a semisynthetic derivative of
camptothecin, is a topoisomerase-I inhibitor, which is active
against a variety of solid tumors, including advanced colorectal,
pulmonary, gastric and ovarian cancer (1,2).
Irinotecan is a prodrug, which is hydrolyzed by liver
carboxylesterase to produce the active metabolite SN-38 (3). SN-38 is eliminated by glucuronidation,
which depends on hepatic UDP glucuronosyltransferase family 1,
member A1 cluster (UGTA1) enzymes (4). Genotype UGT1A1*28 has been found to be
associated with decreased SN-38 glucuronidation; thus,
irinotecan-induced diarrhea and neutropenia may be increased in
patients with the UGT1A1*28/*28 genotype (5). While several UGT1A1 genotype-directed
administration schedules of irinotecan are currently under
evaluation (6–8), the concept of heritable biological
marker-guided dosing is new and requires further evaluation prior
to introduction in clinical practice (9).
Three schedules of irinotecan administration are
currently in clinical use, namely weekly, bi-weekly and tri-weekly
schedules, among which administration once every 3 weeks and a
weekly 90-min infusion are the ones most commonly used and compared
(10,11). The dose-limiting side effects of the
two schedules are neutropenia and diarrhea. Several comparative
clinical trials have been performed to investigate whether the
efficacy of irinotecan is schedule-dependent and others have
suggested that the toxicity profiles may be distinctive for
different schedules irrespective of the genotype (5,12–18). However, these trials have not been
conclusive regarding the differences between the two commonly used
regimens. Therefore, a meta-analysis of all these individual data
is required to determine the differences.
The objective of the present meta-analysis, which
was based on all the data from randomized controlled trials (RCTs),
was to compare the efficacy and toxicity profiles of the two
different schedules of irinotecan, used alone or in combination
with other drugs in the treatment of various solid tumors with the
aim of determining the optimal administration schedule for this
drug.
Materials and methods
Literature search
The electronic databases PubMed, EMBASE and Cochrane
Library were searched for entries of suitable studies available
prior to November, 2015 using the following search terms:
(irinotecan OR CPT-11 OR Campto OR Camptosar) AND (administration
OR dosage OR schedule OR regimen OR weekly). There were no language
or publication status restrictions.
Inclusion criteria
Patients who were histologically or cytologically
diagnosed with solid carcinomas and who had received irinotecan,
alone or in combination with other chemotherapeutic drugs, were
included.
Measures of outcome
The objective response rate (ORR), median time to
progression (TTP), overall survival (OS) and the incidence of
adverse effects, including neutropenia and diarrhea, were assessed
in the present study.
Regimens
The regimens compared in the present meta-analysis
were 3-weekly vs. weekly irinotecan for the treatment of solid
tumors.
Data extraction
The titles and abstracts of all identified trials
were screened by two authors independently for inclusion criteria.
Disagreements were resolved by consensus. The same two authors
extracted data independently using standard data extraction
forms.
Quality assessment of the studies
The quality of the studies was assessed by two
independent authors. Discrepancies were resolved by discussion with
another author. Quality was assessed based on randomization,
allocation concealment, blinding (participants, investigators,
outcome assessors and data analysis), loss to follow-up and
intent-to-treat (ITT) analysis. The trials were graded as A, B or C
following the criteria of Cochrane with the aim of assessing all
types of bias (19).
Statistical analysis
Quantitative meta-analyses were performed to compare
the efficacy and adverse effects between the 3-weekly and weekly
groups. Forest plots were generated using Review Manager software
version 5.3 (http://tech.cochrane.org/revman/download). The risk
ratio (RR) was calculated along with its 95% confidence intervals
(CI) for dichotomous data and the standard mean difference (SMD)
was used for continuous outcomes. Statistical heterogeneity between
studies was assessed by means of I2 statistics.
I2<25% was considered to indicate a low level of
heterogeneity, I2=25–50% moderate-level and
I2>50% high-level heterogeneity. A fixed-effects
model was used for calculations if there was no significant
heterogeneity, while a random-effects model was applied if clinical
and methodological heterogeneity were present. All statistical
outcomes were two-sided and the significance threshold was set at
P<0.05.
Results
Study selection
The literature search yielded 1,821 studies, of
which 1,814 were excluded due to irrelevant content, not meeting
the inclusion criteria, repeated content or non-randomization.
Finally, 7 RCTs (12–18), comprising a total of 884 patients,
were included in the present meta-analysis. Among these, 3 RCTs
included regimens in which irinotecan was used in combination with
other therapeutic drugs (12,13,15) and 4
RCTs used irinotecan alone (14,16–18).
Characteristics of included studies
and quality assessment
The characteristics of the studies assessed are
listed in Table I and the results of
quality assessment are shown in Table
II. The overall quality of the studies was moderate to low
(grades B and C). All 7 studies were randomized (12–18).
Bajetta et al (12) used a
computer-generated randomization list, Fuchs et al (14) used electronical randomization, Perez
et al (16) used a dynamic
allocation procedure and Tsavaris et al (18) used closed envelopes, while the method
of randomization was not specified in the remaining 3 studies
(13,15,17). None
of the 7 studies mentioned allocated concealment. One study did not
use blinding (17), while blinding
was not mentioned in the remaining 6 studies (12–16,18). One
study reported on loss to follow-up without ITT analysis (12) and 2 studies reported on loss to
follow-up and performed ITT analysis (14,17), while
the remaining studies did not describe loss to follow-up (13,15,16,18).
 | Table I.Characteristics of included
studies. |
Table I.
Characteristics of included
studies.
| Authors (Refs.) | Group | Tumor type | Treatment | Patients (n) | Administration
schedule |
|---|
| Bajetta et al
(12) | q3w | Metastatic colorectal
carcinoma | First-line | 68 | CPT-11 240–300
mg/m2 d1 + CAP 1,250 mg/m2 d2-15 twice daily,
q3w |
|
| qw |
|
| 66 | CPT-11 120–150
mg/m2 d1,8 + CAP 1,250 mg/m2 d2-15 twice
daily, qw |
| Borner et al
(13) | q3w | Metastatic
colorectal carcinoma | First-line | 37 | CPT-11 240–300
mg/m2 d1 + CAP 1,000 mg/m2 d1-14 twice daily,
q3w |
|
| qw |
|
| 38 | CPT-11 70
mg/m2 d1,8,15 + CAP 1,000 mg/m2 d1-14 twice
daily, qw |
| Fuchs et al
(14) | q3w | Metastatic
colorectal carcinoma | Second-line | 190 | CPT-11 300–350
mg/m2 d1 q3w |
|
| qw |
|
| 94 | CPT-11 125
mg/m2 weekly for 4 weeks followed by a 2-week
interval |
| Mascarenhas et
al (15) | q3w |
Rhabdomyosarcoma | Second-line | 47 | CPT-11 50
mg/m2 d1-5 + vincristine 1.5 mg/m2 d1,8 twice
daily, q3w |
|
| qw |
|
| 42 | CPT-11 20
mg/m2 d1-5 of weeks 1, 2, 4 and 5 + vincristine 1.5
mg/m2 d1,8 twice daily, qw |
| Perez et al
(16) | q3w | Metastatic breast
cancer | Second-line or
beyond | 51 | CPT-11 240
mg/m2 d1 q3w |
|
| qw |
|
| 53 | CPT-11 100
mg/m2 d1 weekly for 4 weeks followed by a 2-week
interval |
| Schoemaker et
al (17) | q3w | Metastatic
colorectal carcinoma | Second-line | 41 | CPT-11 350
mg/m2 d1 q3w |
|
| qw |
|
| 37 | CPT-11 125
mg/m2 d1 weekly for 4 weeks followed by a 2-week
interval |
| Tsavaris et
al (18) | q3w | Advanced colorectal
carcinoma | Second-line | 60 | CPT-11 350
mg/m2 d1 q3w |
|
| qw |
|
| 60 | CPT-11 175
mg/m2 d1,10 qw |
 | Table II.Quality assessment of included
studies. |
Table II.
Quality assessment of included
studies.
| Authors
(Refs.) | Randomization | Allocated
concealment | Blinding | Loss to follow-up
and dropout | Quality grade |
|---|
| Bajetta et
al (12) | Adequate | n.s. | n.s. | Yes without ITT
analysis | B |
| Borner et al
(13) | n.s. | n.s. | n.s. | No | B |
| Fuchs et al
(14) | Adequate | n.s. | n.s. | Yes with ITT
analysis | B |
| Mascarenhas et
al (15) | n.s. | n.s. | n.s. | No | B |
| Perez et al
(16) | Adequate | n.s. | n.s. | No | B |
| Schoemaker et
al (17) | n.s. | n.s. | Not used | Yes with ITT
analysis | C |
| Tsavaris et
al (18) | Adequate | n.s. | n.s. | No | B |
Efficacy
ORR
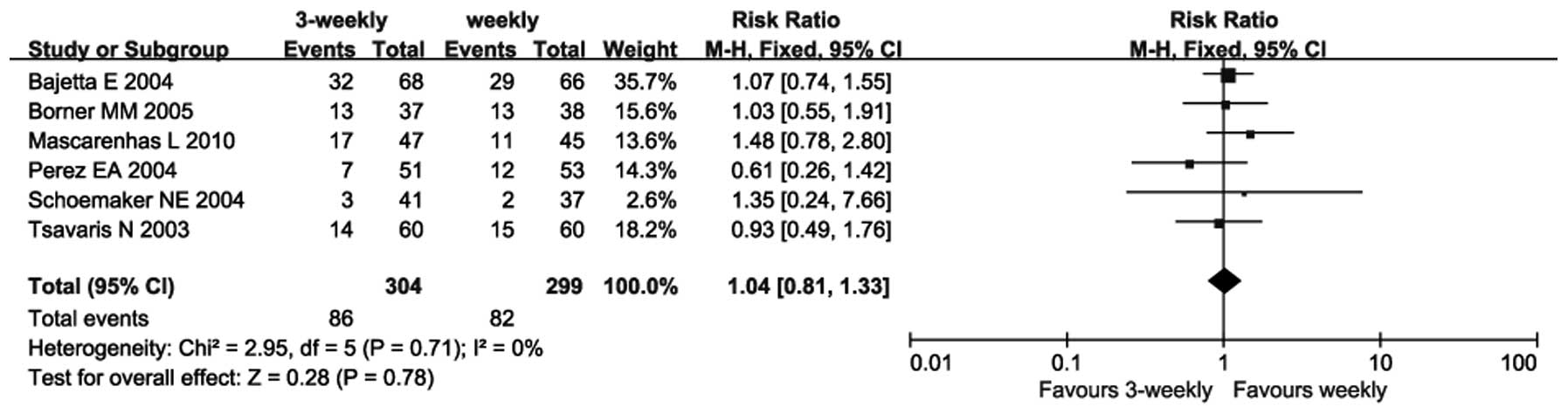
Six trials provided an ORR (12,14,15–18).
As there was no heterogeneity between these trials (P=0.71;
I2=0%), a fixed-effects model was used. The
meta-analysis revealed no significant difference between the
3-weekly and weekly groups regarding ORR (RR=1.04; 95% CI:
0.81–1.33; P=0.78) (Fig. 1).
TTP
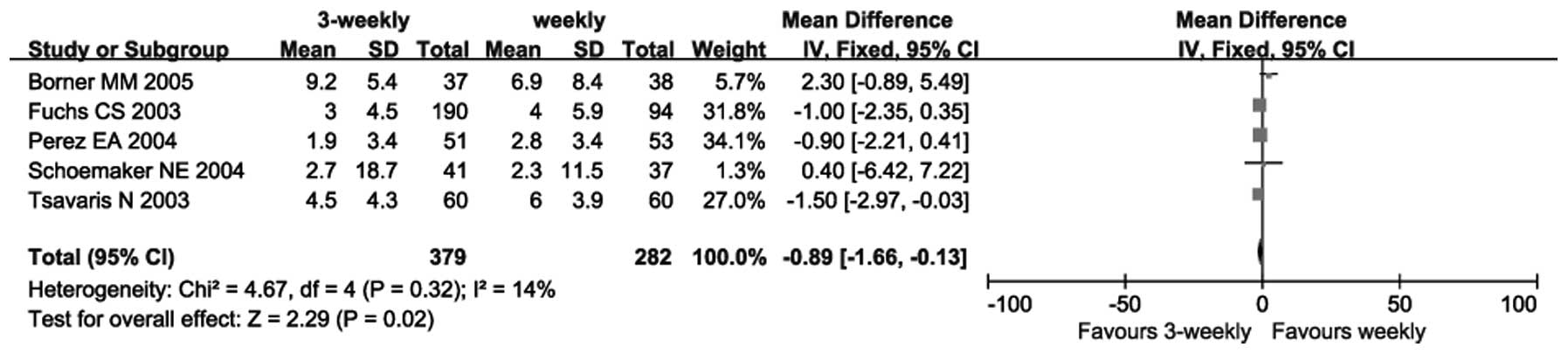
Five trials reported on TTP (13,14,16–18).
As there was no heterogeneity between these trials (P=0.32;
I2=14%), a fixed-effects model was used. The
meta-analysis revealed a significant difference in favor of the
3-weekly group regarding TTP (SMD=−0.89; 95% CI: −1.66 to −0.13);
P=0.02 (Fig. 2).
OS
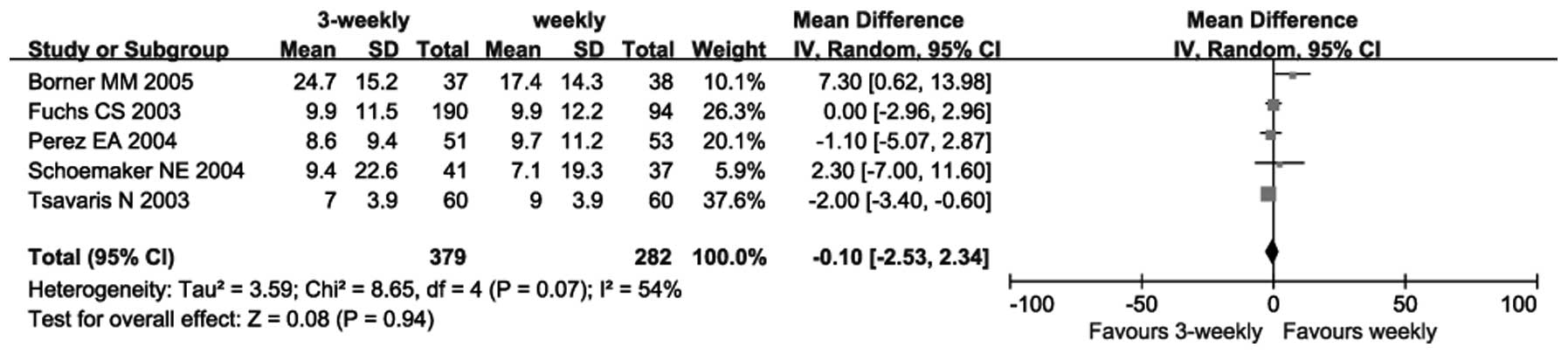
Five trials (13,14,16–18)
reported on OS. Due to heterogeneity among these trials (P=0.07;
I2=54%), a random-effects model was used. The
meta-analysis revealed no significant difference between the
3-weekly and weekly groups (SMD=−0.10, 95% CI: −2.53 to 2.34,
P=0.94) (Fig. 3).
Adverse effects
Diarrhea
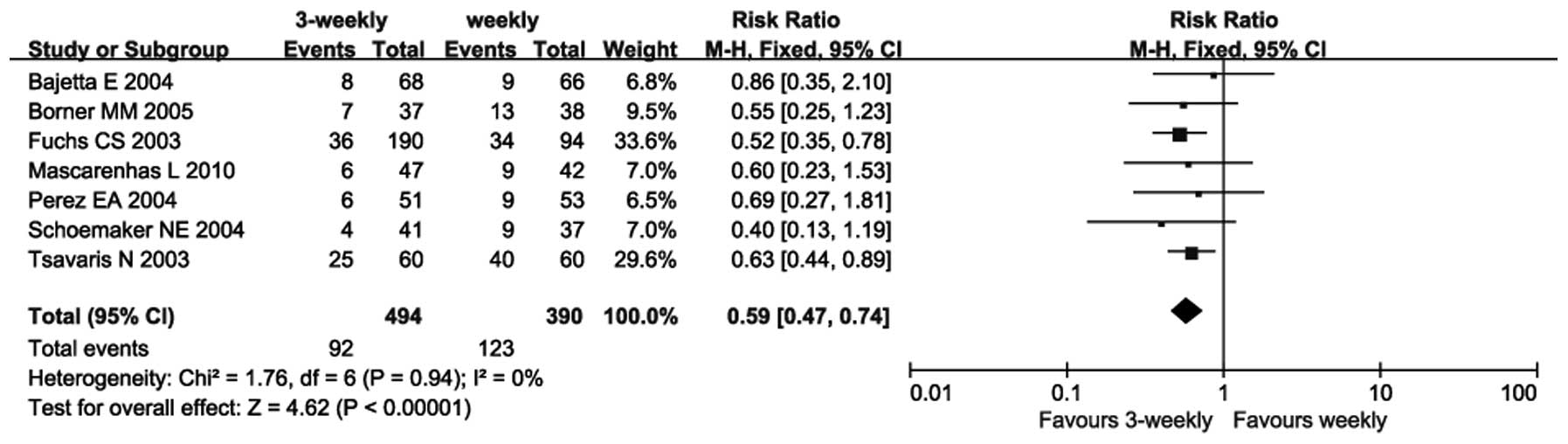
All 7 trials (12–18)
reported on the incidence of diarrhea. As there was no
heterogeneity between the trials (P=0.94; I2=0%), a
fixed-effects model was used. The meta-analysis revealed that the
incidence of diarrhea in the 3-weekly group was significantly lower
compared with that in the weekly group (RR=0.59; 95% CI: 0.47–0.74;
P<0.00001) (Fig. 4).
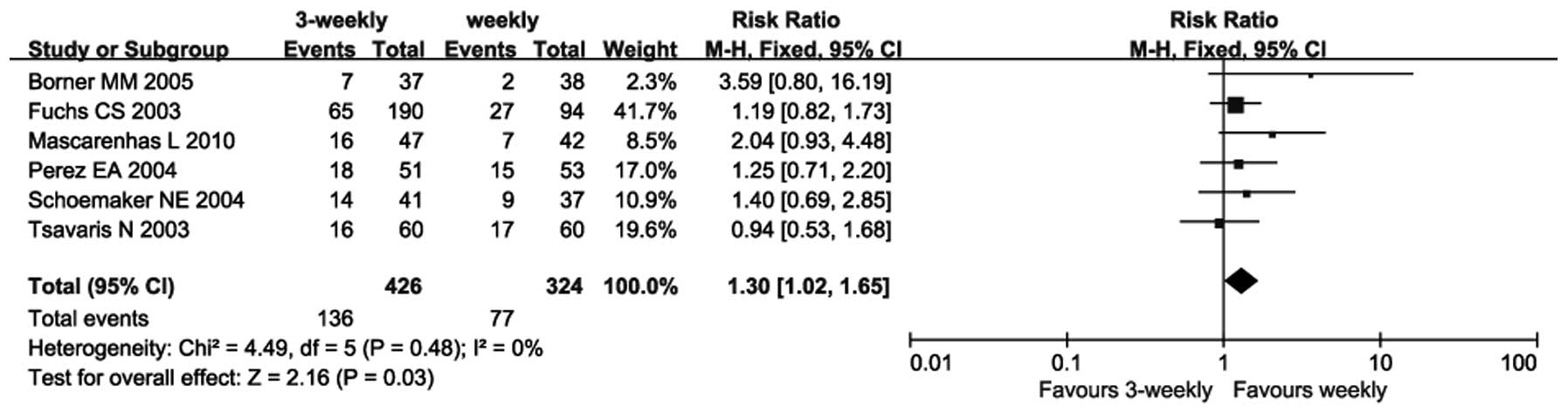
Neutro penia
Six trials reported on the incidence of neutropenia
(13–18). As there was no heterogeneity between
these trials (P=0.48; I2=0%), a fixed-effects model was
used. The meta-analysis revealed that the incidence of neutropenia
in the 3-weekly group was significantly higher compared with that
in the weekly group (RR=1.30; 95% CI: 1.02–1.65; P=0.03) (Fig. 5).
Discussion
The results of the present meta-analysis revealed
that the 3-weekly and weekly regimens of irinotecan administration
had a similar efficacy interms of ORR and OS, while the TTP tended
to be longer with the 3-week regimen. Furthermore, the 3-weekly
group had a lower incidence of grade 3/4 diarrhea compared with the
weekly group, while the incidence of grade 3/4 neutropenia was
higher in the 3-weekly group.
Irinotecan is a widely used chemotherapeutic drug
that is effective against several solid tumors, with a single-agent
response rate of 12–50% (20,21). The primary toxicities of irinotecan
are diarrhea and neutropenia, the severity of which has been shown
to be partly associated with UGT1A1*28, a germline genetic variant
affecting the elimination of SN-38. Several trials and a
meta-analysis demonstrated that the UGT1A1*28/*28 genotype is
associated with an increased risk of neutropenia and diarrhea, and
that this association was dose-dependent (5,22).
Genotype-directed dosing has been investigated by a series of
studies (6–8); however, its integration into the
clinical practice remains scant and this drug is still dosed by
body surface area according to almost all guidelines. Furthermore,
SN-38 accounts for only 14% of the total interindividual
variability in the absolute neutrophil count nadir (9), suggesting that additional factors may
lead to neutropenia. Among the factors contributing to
irinotecan-related toxicity, schedule-dependent toxicity has been
most reliably confirmed (23). The
present meta-analysis suggested that the toxicity patterns of the
two different schedules were somewhat distinctive. The 3-weekly
regimen was associated with a lower incidence of diarrhea but a
higher rate of neutropenia compared with the weekly regimen.
Furthermore, the 3-weekly regimen was superior in terms of TTP,
although the OS was similar between the two regimens. Thus, the
irinotecan treatment schedule should be selected according to the
characteristics, physical status, convenience and preference of
each patient.
The trials assessed in the present meta-analysis
were heterogeneous in terms of OS. Treatment response not only
depends on the chemotherapeutic schedule, but is also tumor
type-dependent. Among the included trials, 5 investigated advanced
or metastatic colorectal carcinoma (12–14,17,18),
1 investigated rhabdomyosarcoma (15)
and 1 was on breast cancer (16).
Furthermore, in 2 of the studies, irinotecan was used as first-line
therapy in combination with capecitabine (12,13), while
in the remaining studies, irinotecan monotherapy was used as a
second- or further-line treatment. Moreover, OS tends to be
affected by the subsequent treatment and several other unforeseen
factors. Therefore, the differences in tumor type, treatment
modality/schedule and patient characteristics may all contribute to
the heterogeneity observed. In this context, ORR and TTP may
reflect the acute efficacy of a therapy more accurately, in which
heterogeneity was acceptable or absent. Mascarenhas et al
(15) investigated rhabdomyosarcoma
patients aged <21 years; therefore, a sensitivity analysis was
performed. The result demonstrated that there was no difference in
the overall effect with or without this trial.
The quality of the studies included in the present
meta-analysis was relatively low, which may limit the reliability
of the conclusions. Three trials did not report the details of
randomization (13,15,17),
whereas none of the trials specified whether allocated concealment
was performed. Furthermore, 6 of the studies did not mention
blinding, whereas the remaining study specified that blinding was
not performed (17). In addition, 1
study reported loss to follow-up, while no ITT analysis was
performed (12). All these factors
may have led to selection, performance, measurement and attrition
biases. Of the 7 the included trials, 3 were from the USA (14–16) and 4
from Europe (12,13,17,18), which
may reduce the universality of the results. The relatively small
sample size and the fact that most of the studies were relatively
old (>10 years) are also considered as limitations of the
present meta-analysis. Therefore, it is recommended that more RCTs
of high quality from different countries and with improved design
are performed in the future.
In conclusion, the present meta-analysis suggested
that, compared to the weekly regimen of irinotecan, the 3-weekly
regimen yielded a similar ORR and OS, but a longer TTP. The two
regimens exhibited distinctly different toxicity profiles: While
the 3-weekly regimen was associated with a lower incidence of
diarrhea, it had a higher rate of neutropenia compared with the
weekly regimen. Thus, when selecting an irinotecan treatment
schedule, cost-effectiveness, the patients' performance status and
convenience should be taken into consideration.
Glossary
Abbreviations
Abbreviations:
|
ORR
|
objective response rate
|
|
TTP
|
time to progression
|
|
OS
|
overall survival
|
|
RCT
|
randomized controlled trial
|
|
ITT
|
intent-to-treat
|
|
RR
|
risk ratio
|
|
CI
|
confidence interval
|
|
SMD
|
standard mean difference
|
References
|
1
|
Pitot HC, Wender DB, O'Connell MJ,
Schroeder G, Goldberg RM, Rubin J, Mailliard JA, Knost JA, Ghosh C,
Kirschling RJ, et al: Phase II trial of irinotecan in patients with
metastatic colorectal carcinoma. J Clin Oncol. 15:2910–2919.
1997.PubMed/NCBI
|
|
2
|
Vanhoefer U, Harstrick A, Achterrath W,
Cao S, Seeber S and Rustum YM: Irinotecan in the treatment of
colorectal cancer: Clinical overview. J Clin Oncol. 19:1501–1518.
2001.PubMed/NCBI
|
|
3
|
Rivory LP, Bowles MR, Robert J and Pond
SM: Conversion of irinotecan (CPT-11) to its active metabolite,
7-ethyl-10-hydroxycamptothecin (SN-38), by human liver
carboxylesterase. Biochem Pharmacol. 52:1103–1111. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iyer L, King CD, Whitington PF, Green MD,
Roy SK, Tephly TR, Coffman BL and Ratain MJ: Genetic predisposition
to the metabolism of irinotecan (CPT-11). Role of uridine
diphosphate glucuronosyltransferase isoform 1A1 in the
glucuronidation of its active metabolite (SN-38) in human liver
microsomes. J Clin Invest. 101:847–854. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu ZY, Yu Q, Pei Q and Guo C:
Dose-dependent association between UGT1A1*28 genotype and
irinotecan-induced neutropenia: Low doses also increase risk. Clin
Cancer Res. 16:3832–3842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Innocenti F, Schilsky RL, Ramírez J,
Janisch L, Undevia S, House LK, Das S, Wu K, Turcich M, Marsh R, et
al: Dose-finding and pharmacokinetic study to optimize the dosing
of irinotecan according to the UGT1A1 genotype of patients with
cancer. J Clin Oncol. 32:2328–2334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim KP, Kim HS, Sym SJ, et al: A UGT1A1*28
and *6 genotype-directed phase I dose-escalation trial of
irinotecan with fixed-dose capecitabine in Korean patients with
metastatic colorectal cancer. Cancer Chemother Pharmacol.
71:1609–1617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goetz MP, McKean HA, Reid JM, et al:
UGT1A1 genotype-guided phase I study of irinotecan, oxaliplatin,
and capecitabine. Invest New Drugs. 31:1559–1567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phelps MA and Sparreboom A: Irinotecan
pharmacogenetics: A finished puzzle? J Clin Oncol. 32:2287–2289.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rothenberg ML, Eckardt JR, Kuhn JG, Burris
HA III, Nelson J, Hilsenbeck SG, Rodriguez GI, Thurman AM, Smith
LS, Eckhardt SG, et al: Phase II trial of irinotecan in patients
with progressive or rapidly recurrent colorectal cancer. J Clin
Oncol. 14:1128–1135. 1996.PubMed/NCBI
|
|
11
|
Armand JP, Extra YM, Catimel G, Abigerges
D, Marty M and Clavel M: Rationale for the dosage and schedule of
CPT-11 (irinotecan) selected for phase II studies, as determined by
European phase I studies. Ann Oncol. 7:837–842. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bajetta E, Di Bartolomeo M, Mariani L,
Cassata A, Artale S, Frustaci S, Pinotti G, Bonetti A, Carreca I,
Biasco G, et al: Randomized multicenter phase II trial of two
different schedules of irinotecan combined with capecitabine as
first-line treatment in metastatic colorectal carcinoma. Cancer.
100:279–287. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borner MM, Bernhard J, Dietrich D, et al:
A randomized phase II trial of capecitabine and two different
schedules of irinotecan in first-line treatment of metastatic
colorectal cancer: Efficacy, quality-of-life and toxicity. Ann
Oncol. 16:282–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fuchs CS, Moore MR, Harker G, Villa L,
Rinaldi D and Hecht JR: Phase III comparison of two irinotecan
dosing regimens in second-line therapy of metastatic colorectal
cancer. J Clin Oncol. 21:807–814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mascarenhas L, Lyden ER, Breitfeld PP, et
al: Randomized phase II window trial of two schedules of irinotecan
with vincristine in patients with first relapse or progression of
rhabdomyosarcoma: A report from the Children's Oncology Group. J
Clin Oncol. 28:4658–4663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perez EA, Hillman DW, Mailliard JA, Ingle
JN, Ryan JM, Fitch TR, Rowland KM, Kardinal CG, Krook JE, Kugler JW
and Dakhil SR: Randomized phase II study of two irinotecan
schedules for patients with metastatic breast cancer refractory to
an anthracycline, a taxane, or both. J Clin Oncol. 22:2849–2855.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schoemaker NE, Kuppens IE, Moiseyenko V,
Glimelius B, Kjaer M, Starkhammer H, Richel DJ, Smaaland R,
Bertelsen K, Poulsen JP, et al: A randomised phase II multicentre
trial of irinotecan (CPT-11) using four different schedules in
patients with metastatic colorectal cancer. Br J Cancer.
91:1434–1441. 2004.PubMed/NCBI
|
|
18
|
Tsavaris N, Ziras N, Kosmas C, Giannakakis
T, Gouveris P, Vadiaka M, Dimitrakopoulos A, Karadima D, Rokana S,
Papalambros E, et al: Two different schedules of irinotecan
(CPT-11) in patients with advanced colorectal carcinoma relapsing
after a 5-fluorouracil and leucovorin combination. A randomized
study. Cancer Chemother Pharmacol. 52:514–519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
andomized trials. BMJ. 18:d59282011. View Article : Google Scholar
|
|
20
|
Rothenberg ML, Cox JV, DeVore RF,
Hainsworth JD, Pazdur R, Rivkin SE, Macdonald JS, Geyer CE Jr,
Sandbach J, Wolf DL, et al: A multicenter, phase II trial of weekly
irinotecan (CPT-11) in patients with previously treated colorectal
carcinoma. Cancer. 85:786–795. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rougier P, Van Cutsem E, Bajetta E,
Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg
H, Wils J, et al: Randomised trial of irinotecan versus
fluorouracil by continuous infusion after fluorouracil failure in
patients with metastatic colorectal cancer. Lancet. 352:1407–1412.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoskins JM, Goldberg RM, Qu P, Ibrahim JG
and McLeod HL: UGT1A1*28 genotype and irinotecan-induced
neutropenia: Dose matters. J Natl Cancer Inst. 99:1290–1295. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Masuda N, Kudoh S and Fukuoka M:
Irinotecan (CPT-11): Pharmacology and clinical applications. Crit
Rev Oncol Hematol. 24:3–26. 1996. View Article : Google Scholar : PubMed/NCBI
|