Introduction
Microsurgical resection has been the standard
treatment for hypoglossal schwannoma, but it may not always be
feasible, particularly when preservation of neurological function
is desired. Moreover, resection may be associated with unintended
neurological deficits and other potentially severe complications
(1,2).
Therefore, further investigation is required to design a safe and
effective treatment for hypoglossal schwannomas. In this study, we
report our experience with treating hypoglossal schwannomas using
hypofractionated stereotactic radiotherapy.
Case reports
Case 1
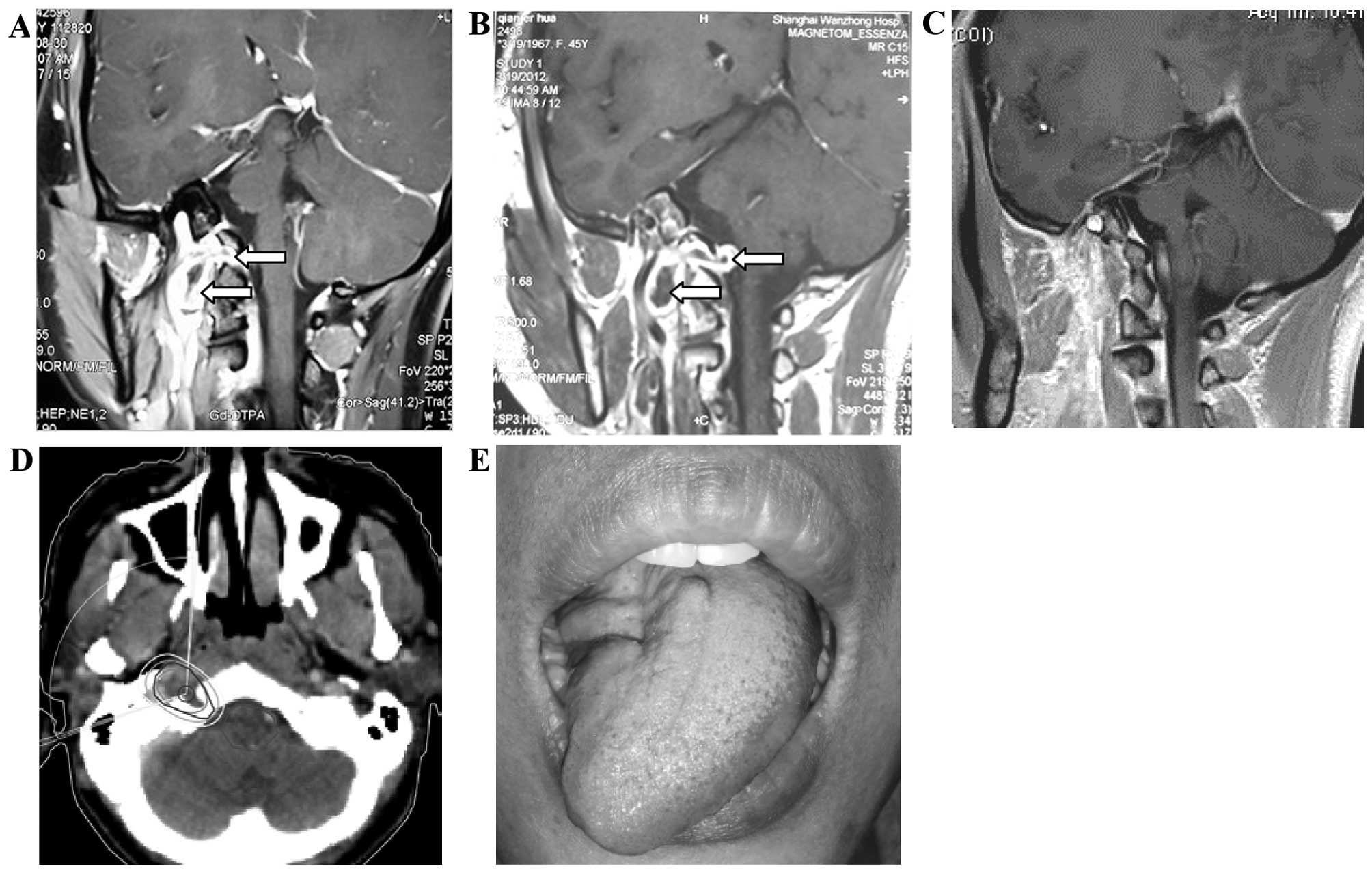
A 33-year-old woman presented with a 2-month history
of intermittent right occipital headache; in addition, her already
existing neck rotation disorder, unclear pronunciation and
dysphagia had gradually worsened over ~3 months. On neurological
examination, the patient was alert and oriented. The cranial nerve
functions were nearly intact, with the exception of a tongue
deviation to the right side and accompanying hemiatrophy. The
muscle strength and tone in all the extremities were normal. The
examination for neurofibromatosis type 2 was negative. The magnetic
resonance imaging (MRI) scan revealed a cystic mass adjacent to the
cranial base and extending into the right hypoglossal canal. The
tumor was dumbbell-shaped, measured ~20×18×42 mm, with distinct
borders. A contrast-enhanced MRI showed diffuse enhancement in the
solid portion of the tumor. The clinical diagnosis was hypoglossal
schwannoma. As the patient refused surgery due to concerns
regarding the risks of the operation, stereotactic radiotherapy
(SGS-I Stereotactic Gamma-Ray system; Huiheng Medical Inc.,
Shenzhen, China) was used to treat the tumor. The gross tumor
volume (GTV) included the intracranial and extracranial lesions. A
margin of 2 mm was added to the GTV to formulate the planning
target volume (PTV). A dose of 30 Gy in fraction sizes of 3 Gy was
delivered to the PTV. The edges of the PTV and GTV were encompassed
by 50% and 70% isodose curves, respectively. The maximum dose to
the spinal cord was 25 Gy. Six months after the treatment, the
patient experienced gradual improvement of the symptoms. The MRI
scan performed 5 years after the stereotactic radiotherapy revealed
that the hypoglossal neurinoma exhibited a complete response. The
patients headaches had disappeared and her neck movements had
become smooth; her pronunciation and eating ability had also
significantly improved (Fig. 1).
Case 2
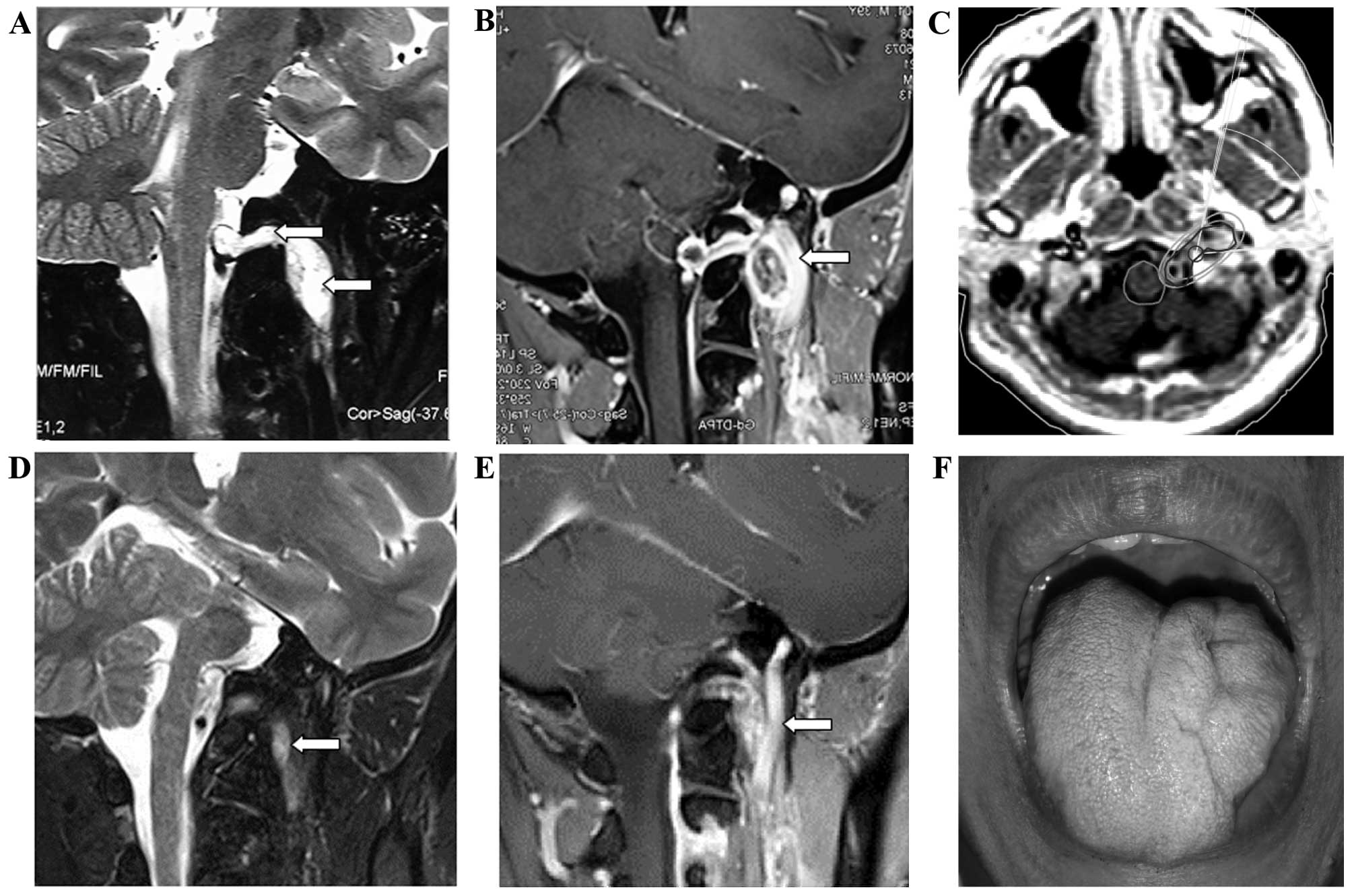
A 38-year-old man presented with throbbing left
occipital headache that had persisted for ~6 months. The pain was
severe at night and affected his ability to sleep. A physical
examination revealed left tongue hemiatrophy, without any clinical
manifestations of neurofibromatosis type 2. The MRI scan revealed a
dumbbell-shaped cystic mass adjacent to the cranial base that
extended into the left hypoglossal canal and measured ~25×20×48 mm.
A contrast-enhanced MRI showed obvious enhancement in the solid
portion of the tumor. The tumor was ultimately diagnosed as
hypoglossal schwannoma. The patient refused surgery and instead
opted for stereotactic radiotherapy. A total dose of 30 Gy in 10
fractions was delivered to the intracranial and extracranial
lesions, with a maximum dose to the spinal cord of 26 Gy. Three
months after the treatment, the patients headaches had gradually
improved. The MRI scan performed 4 years after the treatment
revealed that the hypoglossal neurinoma had decreased in size, and
the patient's headaches had disappeared (Fig. 2).
Discussion
The hypoglossal nerve, which is a motor nerve,
arises from the hypoglossal nucleus in the brainstem and, after
passing through the subarachnoid space, it exits the skull base of
the posterior fossa through the hypoglossal canal. Upon emerging
from the hypoglossal canal, the hypoglossal nerve spirals behind
the vagus nerve and passes between the internal carotid artery and
the internal jugular vein lying on the carotid sheath. After
passing deep into the posterior belly of the digastric muscle, it
reaches and innervates the ipsilateral tongue muscles.
Hypoglossal schwannomas are benign tumors
originating from the Schwann cells of the hypoglossal nerve. These
tumors are extremely rare, constituting only ~1% of all
intracranial schwannomas, as neurinomas develop more often in
sensory rather than motor nerves (3).
To the best of our knowledge, a total of 89 cases have been
reported in the English literature over the last three decades.
Among those cases, the male:female ratio was 2:3 and Asian patients
accounted for 73.0% of the cases (29.2% were from from China, 23.6%
from Japan and 16.8% from India). The patients were aged 9–85
years, with the highest incidence between 30 and 60 years
(73.3%).
Hypoglossal schwannomas may be classified according
to tumor location as follows: Type A, intracranial tumor,
accounting for 31.5% of the cases; type B, both intra- and
extracranial tumor (referred to as dumbbell-shaped tumor),
accounting for 50.0% of the cases; and type C, extracranial-only
tumor, accounting for 18.5% of the cases (4). There is a variety of clinical symptoms
associated with the progress of hypoglossal schwannomas, as the
growth of the tumor compresses the vessels and nerves adjacent to
it. The most common symptom is hypoglossal nerve palsy (93.5%).
Other symptoms include palsies of the lower cranial nerves (50%)
such as the glossopharyngeal and vagus nerves; cerebellar signs
(47.8%); occipital and nuchal pain (54.3%) due to radicular and
meningeal irritation; and long-term signs, such as motor (41.3%)
and sensory disturbances (37.0%) due to compression by the tumor
(5). In the most common schwannomas,
two distinct histological patterns are observed, referred to as
Antoni types A and B. Antoni type A tissues are characterized by
compact Schwann cells with nuclear palisading, whereas Antoni type
B tissues display considerable cell pleomorphism in a loosely
arranged reticular network, often with cystic spaces (6). The cystic spaces may result in high
signal intensity on T2-weighted MRI images. Schwannomas are
typically isointense or mildly hypointense relative to the gray
matter on T1-weighted images. Gadolinium enhancement is typically
homogeneous, whereas some larger schwannomas may include areas of
cystic degeneration and heterogeneous signal intensity; these
findings are based on increased numbers of areas with Antoni type B
histological characteristics. In our two cases, the tumors were
dumbbell-shaped, with intra- and extracranial components, and were
both associated with the typical symptoms and imaging
characteristics of a hypoglossal schwannoma.
As hypoglossal schwannomas are benign, slow-growing
tumors, complete resection may be curative. However, microsurgical
resection has traditionally been associated with a high risk of
postoperative deficits and mortality, particularly in cases of
dumbbell-shaped hypoglossal schwannomas. Over 50% of these patients
succumbed due to respiratory distress within 4 weeks after surgery
(7). However, a better understanding
of the cranial base microanatomy and the operative approaches has
led to the development of the extreme lateral infrajugular
transcondylar-transtubercular exposure (ELITE) technique. This
approach provides sufficient exposure for the majority of
dumbbell-shaped hypoglossal schwannomas (8,9). Nonaka
et al (10) reported 13 cases
of hypoglossal schwannomas that were managed with the ELITE
technique, which is the largest series to date; a gross total
resection of the tumor was achieved in 10 patients, a near-total
tumor resection in 1 patient, and a subtotal resection in 2
patients. However, there was no improvement in the preoperative
cranial nerve deficits in any of the patients following surgery.
Postoperative hoarseness was observed in 4 patients (30.7%),
dysphagia in 3 (23.1%), facial weakness in 1 (7.7%) and
cerebrospinal fluid leaks, which persisted despite the use of
lumbar drains, in 1 patient (7.7%). The results demonstrated that,
although surgery may achieve maximum tumor removal, the benefits
for patients may not be optimal. In the modern era, intracranial
schwannomas are detected at an earlier stage; consequently, they
are usually of small to moderate size at diagnosis, and are
therefore amenable to radiosurgery, which offers a minimally
invasive alternative to microsurgery. Although the literature
reports involve a small number of patients, they shed some light on
the effectiveness and safety of radiosurgery for the treatment of
hypoglossal schwannoma. Kimball et al (11) reported one case treated with linear
accelerator radiosurgery, in which the patient experienced an
improvement in dysphonia. In addition, Elsharkawy et al
(12) reported two cases of
hypoglossal schwannoma that regressed following treatment with
Gamma Knife radiosurgery.
Fractionated radiotherapy has been used in certain
centers for the treatment of non-vestibular schwannomas and has
achieved tumor control similar to that achieved with radiosurgery.
Showalter et al (13) reported
a series of patients who received radiotherapy for non-acoustic
cranial nerve schwannomas (including two cases of hypoglossal
schwannomas); a total of 24 patients received fractionated
radiotherapy, delivered in 1.8- to 2.0-Gy fractions at a median
dose of 50.4 Gy; 15 patients received radiosurgery at a median dose
of 12.0 Gy. The 2-year actuarial tumor control rate following
fractionated radiotherapy and radiosurgery was 95%. Cranial nerve
deficits improved in 50%, remained stable in 46%, and worsened in
4% of the patients. No significant difference was observed between
fractionated radiotherapy and radiosurgery in terms of local
control or improvement in the cranial nerve-related symptoms.
To the best of our knowledge, hypofractionated
stereotactic radiotherapy has not been previously reported in the
treatment of hypoglossal schwannoma. The two cases reported herein
demonstrate that this technique is safe and effective. The total
dose of 30 Gy in 10 3-Gy fractions may be the optimal dosage.
Microsurgical resection remains an important tool in
the treatment of hypoglossal schwannoma, particularly for patients
with giant tumors or those with more severe symptoms. However,
hypofractionated stereotactic radiotherapy or radiosurgery may be a
more valuable treatment option for patients with small tumors or
those with milder symptoms, and it is particularly suitable for
postoperative residual lesions.
References
|
1
|
Sarma S, Sekhar LN and Schessel DA:
Nonvestibular schwannomas of the brain: A 7-year experience.
Neurosurgery. 50:437–448; discussion 438–439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishioka K, Abo D, Aoyama H, Furuta Y,
Onimaru R, Onodera S, Sawamura Y, Ishikawa M, Fukuda S and Shirato
H: Stereotactic radiotherapy for intracranial nonacoustic
schwannomas including facial nerve schwannoma. Int J Radiat Oncol
Biol Phys. 75:1415–1419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heiroth HJ, Riemenschneider MJ, Steiger HJ
and Hänggi D: A cylindrical extracranial cranial base neurinoma of
the hypoglossal nerve: A rare tumor with a rare localization: Case
report. Neurosurgery. 65:E212–E213; discussion E213. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoshi M, Yoshida K, Ogawa K and Kawase T:
Hypoglossal neurinoma - two case reports. Neurol Med Chir (Tokyo).
40:489–493. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato M, Kanai N, Fukushima Y, Matsumoto S,
Tatsumi C, Kitamura K, Ozaki M and Hayakawa T: Hypoglossal
neurinoma extending intra- and extracranially: Case report. Surg
Neurol. 45:172–175. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jadwani S, Bansod S and Mishra B:
Intraoral schwannoma in retromolar region. J Maxillofac Oral Surg.
11:491–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Williams JM and Fox JL: Neurinoma of the
intracranial portion of the hypoglossal nerve. Review and case
report. J Neurosurg. 19:248–250. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sen CN and Sekhar LN: An extreme lateral
approach to intradural lesions of the cervical spine and foramen
magnum. Neurosurgery. 27:197–204. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertalanffy H and Seeger W: The
dorsolateral, suboccipital, transcondylar approach to the lower
clivus and anterior portion of the craniocervical junction.
Neurosurgery. 29:815–821. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nonaka Y, Grossi PM, Bulsara KR, Taniguchi
RM, Friedman AH and Fukushima T: Microsurgical management of
hypoglossal schwannomas over 3 decades: A modified grading scale to
guide surgical approach. Neurosurgery. 69(2 Suppl Operative):
ons121–140; discussion ons140. 2011.PubMed/NCBI
|
|
11
|
Kimball MM, Foote KD, Bova FJ, Chi YY and
Friedman WA: Linear accelerator radiosurgery for nonvestibular
schwannomas. Neurosurgery. 68:974–984; discussion 984.
2011.PubMed/NCBI
|
|
12
|
Elsharkawy M, Xu Z, Schlesinger D and
Sheehan JP: Gamma Knife surgery for nonvestibular schwannomas:
Radiological and clinical outcomes. J Neurosurg. 116:66–72. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Showalter TN, Werner-Wasik M, Curran WJ
Jr, Friedman DP, Xu X and Andrews DW: Stereotactic radiosurgery and
fractionated stereotactic radiotherapy for the treatment of
nonacoustic cranial nerve schwannomas. Neurosurgery. 63:734–740.
2008. View Article : Google Scholar : PubMed/NCBI
|