Introduction
Tsumura-Suzuki obese diabetic (TSOD) mice are an
inbred ddY strain that displays spontaneous metabolic syndrome and
type 2 diabetes mellitus. At the age of ≥8 weeks, this strain
develops apparent obesity, glycosuria, hyperglycemia and
hyperinsulinemia (1–3). Due to its characteristics, the
pathophysiology of TSOD mice is also considered to be reflected in
metabolic syndrome, which is a severe risk factor for the
development of incurable diseases that affect the entire body. From
4 months of age onwards, the liver of TSOD mice starts to exhibit
fatty degeneration, hepatocellular ballooning, Mallory bodies and
neutrophil infiltration, which are markers of non-alcoholic fatty
liver disease (NAFLD) and subsequent non-alcoholic steatohepatitis
(NASH). Furthermore, after 10 months of age, spontaneous hepatic
tumors also start to develop in TSOD mice (4).
NAFLD and NASH are associated with metabolic
syndrome, obesity, type 2 diabetes and dyslipidemia. NASH is
involved in a multistep process that begins with the accumulation
of lipids in the liver and additional factors, such as oxidative
stress and cytokines (5). It has been
reported that NAFLD and NASH may lead to cirrhosis and the
development of hepatic tumors, as well as hepatitis B virus (HBV)
and hepatitis C virus (HCV) infections (6,7). It is
widely believed that the incidence of NAFLD/NASH as a cause of
hepatic tumors will increase with the improvement in anti-HBV and
anti-HCV strategies over time. Hence, thorough understanding of the
pathological sequence from NAFLD/NASH to hepatic tumorigenesis is
required.
A variety of diagnostic markers for hepatocellular
carcinoma (HCC) have been recently identified. Glutamine synthetase
(GS) is one of the markers involved in nitrogen homeostasis in the
liver (8–10). GS is a target of Wnt/β-catenin
signaling, which is activated in HCC, and accounts for the
association between GS expression and the growth of HCC (11,12). We
previously reported that GS-positive lesions were also found in
tumors in TSOD mice (4); however, the
detailed characteristics of GS-positive and -negative tumors in
TSOD mice have not yet been elucidated. The aim of this study was
to clarify the histopathological characteristics of hepatic tumors
derived from TSOD mice in GS-positive and -negative legions.
Materials and methods
TSOD mice
A total of 40 7-week old male TSOD mice were
purchased from the Institute for Animal Reproduction (Kasumigaura,
Ibaraki, Japan). All the mice already exhibited obesity at 7 weeks
of age (>40 g), eventually reaching a weight of >55 g. The
mice were maintained on an MF basal diet (Oriental Yeast, Tokyo,
Japan) and chlorinated water ad libitum, and were housed under
specific pathogen-free conditions. This study was performed in
accordance with the animal experiment guidelines specified by the
University of Toyama. The TSOD mice were sacrificed at 15 months of
age with sodium pentobarbital, and their livers were excised for
histological analysis. During the autopsies, the macroscopic tumors
in the liver were counted and their diameters measured. The excised
livers were then fixed in 10% neutral-buffered formalin and
embedded in paraffin.
Histopathological and
immunohistochemical analysis
All the formalin-fixed, paraffin-embedded tissues
were processed, and 4-µm serial sections were cut and stained with
hematoxylin and eosin. Immunohistochemical staining for GS,
β-catenin and liver fatty acid-binding protein (L-FABP) were also
performed. Rabbit polyclonal anti-GS (clone GS-6; dilution 1:500;
cat. no. MAB302; Millipore, CA, USA), anti-L-FABP (dilution 1:100;
cat. no. ab7366; Abcam, Cambridge, UK) and anti-β-catenin (dilution
1:100; cat. no. GWB-764147; GenWay Biotech, San Diego, CA, USA)
were employed as the primary antibodies. The sections were
incubated with the primary antibodies in a wet chamber for 60 min
at room temperature. After rinsing with Tris-buffered saline (TBS)
containing 0.1% Tween (TBS-T), the sections were incubated with
EnVision Peroxidase (PO) (Dako, Tokyo, Japan) for 60 min at room
temperature. After rinsing in TBS-T, 3,3′-diaminobenzidine (Sigma,
Steinheim, Germany) was applied as a substrate for the PO. Using
GS-stained slides, the diameters of the GS-positive or -negative
tumor lesions were measured.
Statistical analysis
A two-tailed Mann-Whitney U test was employed to
compare the mean diameters of the GS-positive and -negative tumor
lesions. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Macroscopic findings of hepatic tumors
in TSOD mice
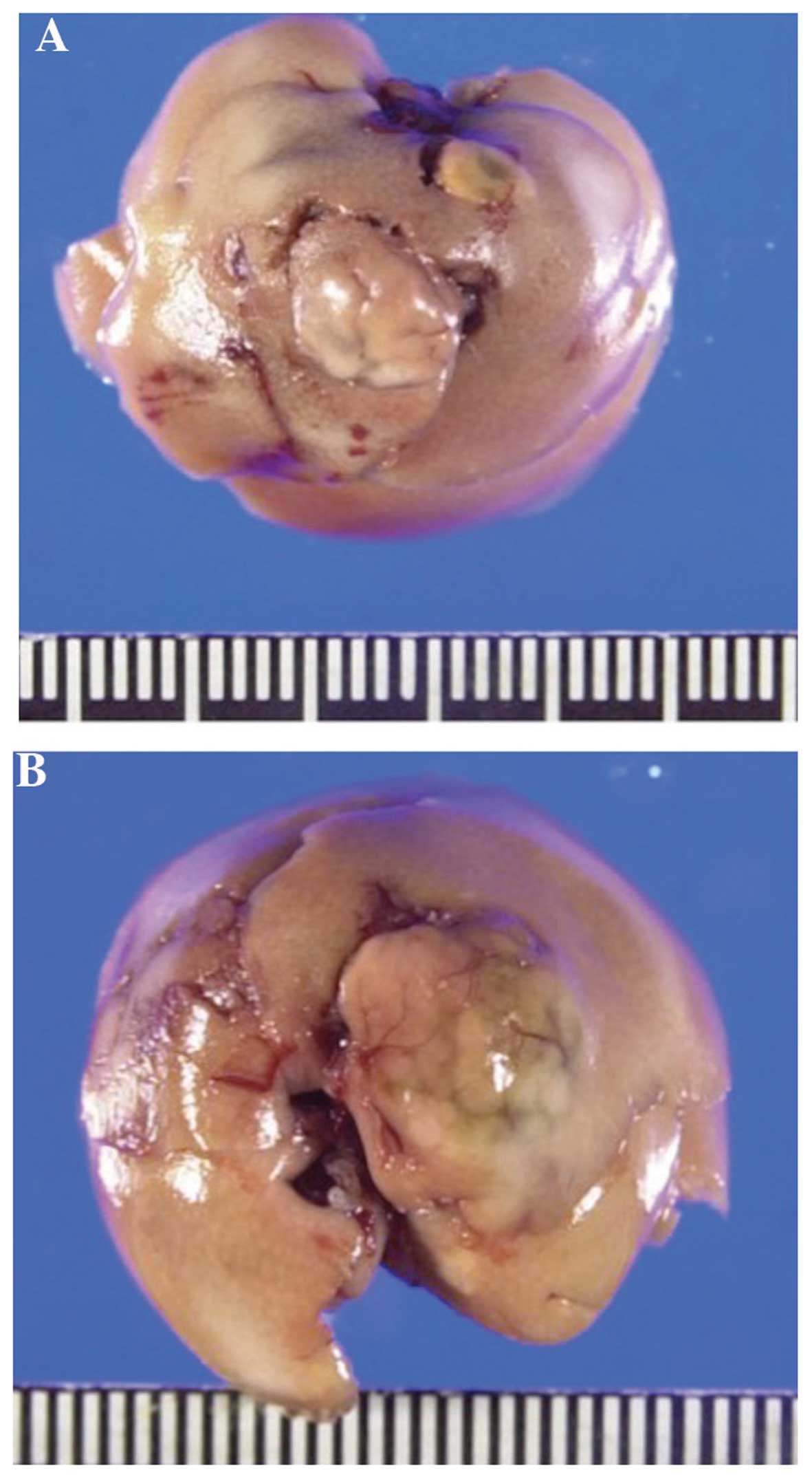
The macroscopic observations of hepatic tumors in
TSOD mice were reported in our previous study (4). Representative pictures of the hepatic
tumors are shown in Fig. 1. In this
experiment, 92.5% (37/40) of the mice exhibited ≥1 hepatic tumors,
with a total of 39 visible tumors. We then measured the macroscopic
size of these tumors; the mean diameter ± standard deviation was
5.3±2.8 mm (data not shown).
Characteristics of GS-positive and
-negative tumors in TSOD mice
Using all the tumor lesions, we conducted
immunohistochemical staining for GS. The majority (28/39; 71.8%) of
the tumors expressed GS in a diffuse manner. Tumors with >50%
stained cells of any intensity were classified as GS-positive. A
typical image of a GS-positive tumor was shown in our previous
study (4). The mean diameters of the
GS-positive and -negative tumors were 4.55±3.07 mm and 3.59±0.98
mm, respectively; the difference was not statistically significant
(Table I).
 | Table I.Size of glutamine synthetase
(GS)-positive and GS-negative liver tumors in Tsumura-Suzuki obese
diabetic mice. |
Table I.
Size of glutamine synthetase
(GS)-positive and GS-negative liver tumors in Tsumura-Suzuki obese
diabetic mice.
| Tumor type | No. of tumors | Mean diameter ±
standard deviation (mm) | P-value |
|---|
| GS-positive | 28 | 4.55±3.07 | 0.708 |
| GS-negative | 11 | 3.59±0.98 |
|
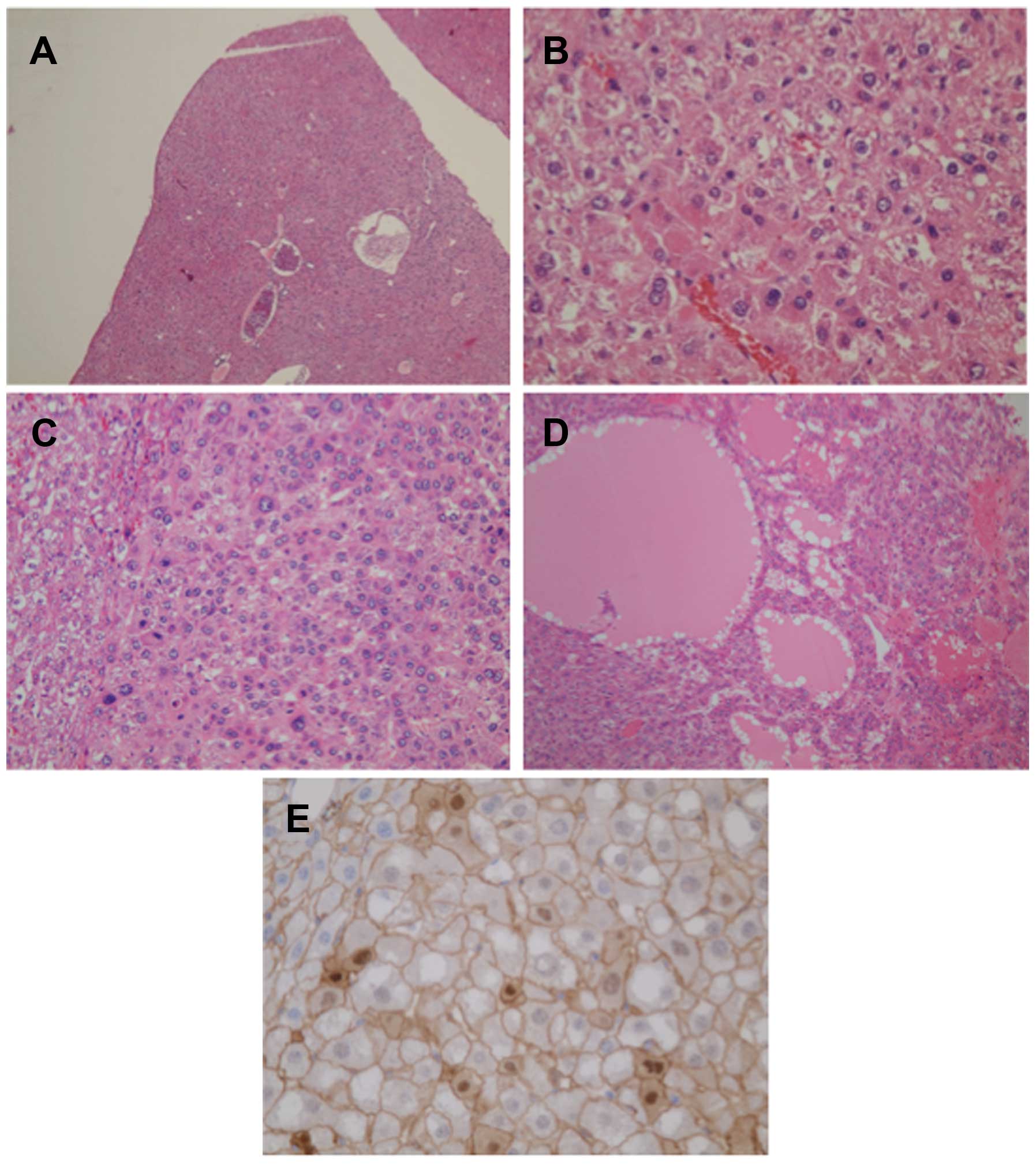
Histologically, small lesions (<1 mm) in
GS-positive cases exhibited dysplastic nodules with nuclear atypia
(Fig. 2A and B). By contrast, part of
the large lesions (>3 mm) of the GS-positive tumors exhibited a
thick trabecular pattern (Fig. 2C)
and/or a pseudoglandular pattern (Fig.
2D), resembling that of human HCC. When testing GS-positive
tumors for β-catenin via immunostaining, translocation of β-catenin
into nuclei with enhanced membranous expression was found in a
measurable population of tumor cells (Fig. 2E). Regardless of the tumor size,
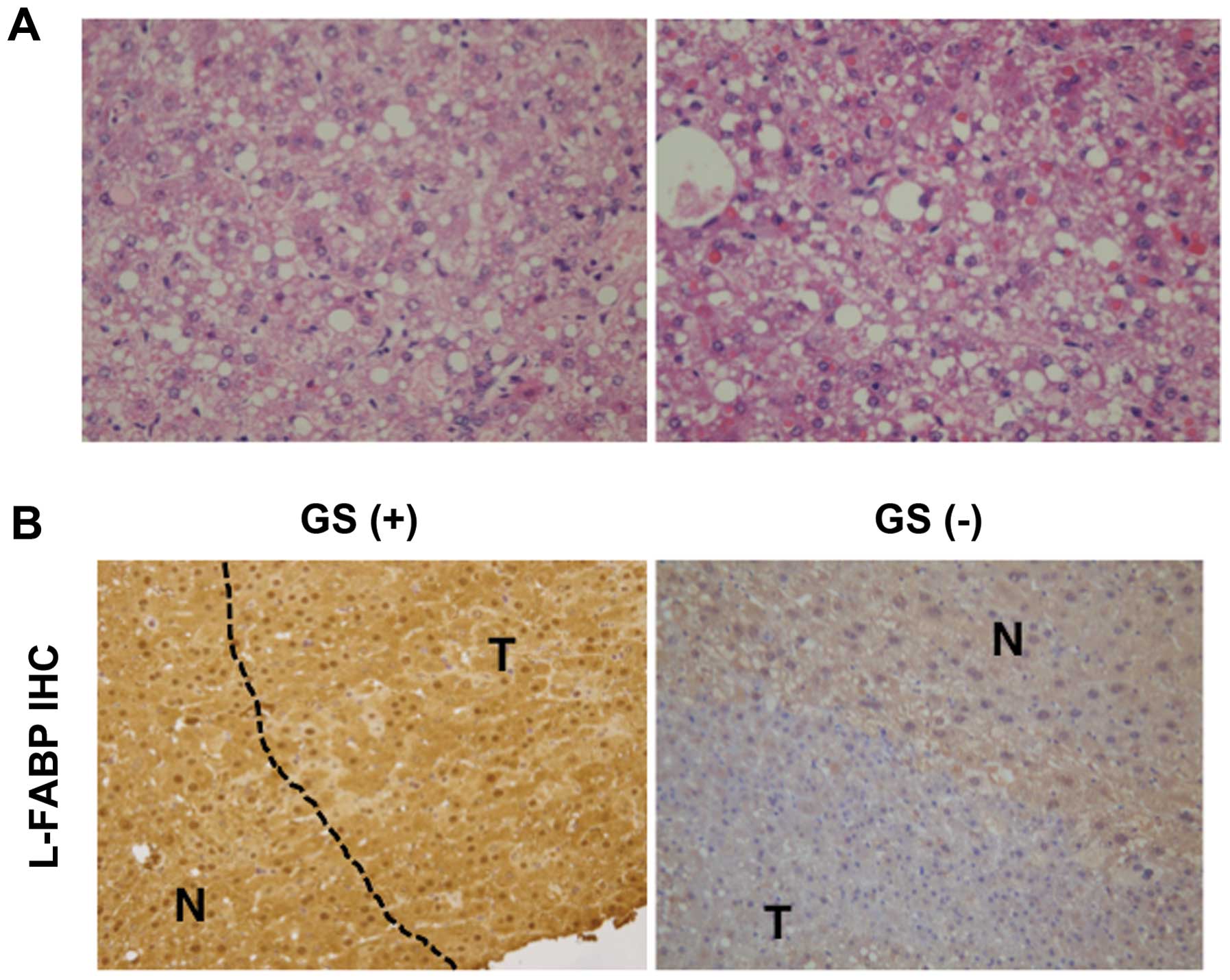
GS-negative tumors exhibited profound fatty change with low nuclear
atypia (Fig. 3A). Since this is
similar to benign hepatocellular adenoma (HCA), we next performed
immunohistochemical staining for L-FABP, a target of hepatocyte
nuclear factor (HNF) 1α. The GS-positive tumor lesions expressed
L-FABP at levels similar to those in the adjacent normal areas,
whereas expression of L-FABP in GS-negative tumors was apparently
lower compared with that in adjacent normal areas (Fig. 3B).
Discussion
Several researchers have focused on the pathological
process from NAFLD/NASH to hepatic tumorigenesis and the
consequences of this process, as elucidating these will enable an
understanding of the molecular mechanisms involved and, thus,
planning of an effective treatment strategy. Based on their
clinical characteristics, the association between NAFLD/NASH and
hepatic tumorigenesis has been extensively discussed (13); however, the molecular basis of this
correlation has not yet been clearly determined. The use of an
appropriate animal model is a potent methodology to enable the
molecular analysis of this pathogenetic process. Thus, TSOD mice,
which exhibit typical symptoms of the metabolic
syndrome-NAFLD/NASH-hepatic tumor sequence, are an ideal model for
analyzing the pathological processes from NAFLD/NASH to hepatic
tumorigenesis (14). TSOD mice
spontaneously develop hepatic tumors at 15 months of age at an
extraordinarily high rate compared with other experimental animal
models. On average, these tumors are sufficiently large to be
detected with a naked eye and, thus, the tumor lesions alone may be
isolated for further analysis. This characteristic provides major
advantages for biochemical or biomolecular examinations.
To assess whether tumors from TSOD mice exhibit the
characteristics of HCC, we performed immunohistochemical staining
for GS, which is a diagnostic marker of HCC in humans. Over 70% of
the tumors were positive for GS in a diffuse manner. Small lesions
in GS-positive cases exhibited dysplastic nodules, which are
considered to be the premalignant lesions of liver cancer. In
larger GS-positive lesions, thick trabecular and pseudoglandular
patterns were identified, partially invading the portal area. The
early changes (after 6 months of age) observed in the liver of TSOD
mice include hepatocellular ballooning and Mallory-Denk bodies
(data not shown). The pathological findings described above are
typical of well-differentiated HCC developing in a background of
NASH in human patients. These results strongly suggest that
GS-positive tumors in TSOD mice histologically reflect HCC in
humans. Immunohistochemical staining for β-catenin also
demonstrated that activation of Wnt/β-catenin signaling occurred in
GS-positive tumors. Wnt/β-catenin signaling is closely associated
with malignant transformation and the development of HCC (15,16).
Hence, GS-positive tumors in TSOD mice may exhibit dysplastic
nodule-carcinoma sequences.
Nearly 30% of the tumors in this study were negative
for GS. The majority of the tumors exhibited profound fatty change
and low nuclear grade, whereas some tumors were diagnosed as HCA,
which is a benign liver neoplasm. As shown in Fig. 2A, GS-negative tumors exhibited macro-
and microvesicular steatosis, which are characteristics of human
HCA carrying HNF1α inactivations (17). HNF1α is known as a regulator of
L-FABP, which is expressed in very high levels in the liver
(18,19). Clinically, downregulation of L-FABP,
possibly due to the inactivation of HNF1α, is found at very low
rates in HCC lesions (20).
Immunohistochemical staining for L-FABP revealed that GS-negative
tumors were also negative for L-FABP, whereas GS-positive tumors
expressed high levels of L-FABP, similar to those in the adjacent
normal areas. Moreover, no inflammatory lesions were found in
GS-negative tumors (data not shown), which is another method of
distinguishing malignancy from HCA. Taken together, the
characteristics of GS-negative tumors in TSOD mice indicate that
benign liver neoplasms mimic HNF1α-inactivated HCA, rather than
HCC.
The results of this study suggest that the TSOD
strain is a spontaneous hepatocarcinogenesis model that may be used
to monitor the pathophysiological characteristics of the early to
the well-differentiated stages of HCC. For HCC to develop, no
specific conditions (e.g., gene modification, special diet, or
administration of carcinogenic agents) are required. Furthermore,
GS-positive tumors in TSOD mice possess characteristics similar to
those of well-differentiated HCC in humans. These characteristics
may be valuable for medical or pharmaceutical approaches to
hepatocarcinogenesis, particularly for the examination of molecular
basis and drug effectiveness. However, some issues remain to be
resolved in this model. First, the sequence from NAFLD/NASH to
hepatic tumorigenesis in TSOD mice did not progress through
cirrhosis, which is a major cause of HCC. For example, comparison
of gene expression or metabolic status using the liver from the
carbon tetrachloride-induced cirrhosis model (21) may help us understand why TSOD mice do
not develop cirrhosis. Second, all the tumors examined in this
study were primary tumors, and there were no intrahepatic,
lymphatic, or hematogenous metastases. This may be due to our
observation endpoint and a longer observation period may result in
metastatic lesions in TSOD mice; these issues should be resolved
fairly quickly. In conclusion, as a model of hepatocarcinogenesis,
TSOD mice are a valuable tool for the investigation of HCC, as they
exhibit the specific characteristics of human HCC.
Acknowledgements
We would like to thank Emu Oda, Megimi Kume, Hitomi
Umemoto and Yuuki Morimoto for their help and technical assistance
during the experiments. We would also like to thank Yukari Inoue
for her support during the preparation of this manuscript. The
present study was supported in part by the TSOD Mouse Research Fund
and JSPS KAKENHI (grant nos. 25930018, 15H00465, 15K15098, 25340121
and 24390181).
References
|
1
|
Miura T, Suzuki W, Ishihara E, Arai I,
Ishida H, Seino Y and Tanigawa K: Impairment of insulin-stimulated
GLUT4 translocation in skeletal muscle and adipose tissue in the
Tsumura Suzuki obese diabetic mouse: A new genetic animal model of
type 2 diabetes. Eur J Endocrinol. 145:785–790. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki W, Iizuka S, Tabuchi M, Funo S,
Yanagisawa T, Kimura M, Sato T, Endo T and Kawamura H: A new mouse
model of spontaneous diabetes derived from ddY strain. Exp Anim.
48:181–189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi A, Tabuchi M, Suzuki W, Iizuka
S, Nagata M, Ikeya Y, Takeda S, Shimada T and Aburada M: Insulin
resistance and low sympathetic nerve activity in the Tsumura Suzuki
obese diabetic mouse: A new model of spontaneous type 2 diabetes
mellitus and obesity. Metabolism. 55:1664–1669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishida T, Tsuneyama K, Fujimoto M, Nomoto
K, Hayashi S, Miwa S, Nakajima T, Nakanishi Y, Sasaki Y, Suzuki W,
et al: Spontaneous onset of nonalcoholic steatohepatitis and
hepatocellular carcinoma in a mouse model of metabolic syndrome.
Lab Invest. 93:230–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ono M, Okamoto N and Saibara T: The latest
idea in NAFLD/NASH pathogenesis. Clin J Gastroenterol. 3:263–270.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhala N, Jouness RI and Bugianesi E:
Epidemiology and natural history of patients with NAFLD. Curr Pharm
Des. 19:5169–5176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ratziu V, Bonyhay L, Di Martino V,
Charlotte F, Cavallaro L, Sayegh-Tainturier MH, Giral P, Grimaldi
A, Opolon P and Poynard T: Survival, liver failure and
hepatocellular carcinoma in obesity-related cryptogenic cirrhosis.
Hepatology. 35:1485–1493. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tremosini S, Forner A, Boix L, Vilana R,
Bianchi L, Reig M, Rimola J, Rodríguez-Lope C, Ayuso C, Solé M and
Bruix J: Prospective validation of an immunohistochemical panel
(glypican 3, heat shock protein 70 and glutamine synthetase) in
liver biopsies for diagnosis of very early hepatocellular
carcinoma. Gut. 61:1481–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellamy CO, Maxwell RS, Prost S, Azodo IA,
Powell JJ and Manning JR: The value of immunophenotyping
hepatocellular adenomas: Consecutive resections at one UK centre.
Histopathology. 62:431–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Webb JT and Brown GW Jr: Glutamine
synthetase: Assimilatory role in livers as related to urea
retention in marine chondrichthyes. Science. 208:293–295. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng G, Apte U, Cieply B, Singh S and
Monga SP: siRNA-mediated beta-catenin knockdown in human hepatoma
cells results in decreased growth and survival. Neoplasia.
9:951–959. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lachenmayer A, Alsinet C, Savic R,
Cabellos L, Toffanin S, Hoshida Y, Villanueva A, Minguez B, Newell
P, Tsai HW, et al: Wnt-pathway activation in two molecular classes
of hepatocellular carcinoma and experimental modulation by
sorafenib. Clin Cancer Res. 18:4997–5007. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duan XY, Zhang L, Fan JG and Qiao L: NAFLD
leads to liver cancer: Do we have sufficient evidence? Cancer Lett.
345:230–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakanishi Y, Tsuneyama K, Nomoto K, et al:
Nonalcoholic steatohepatitis and hepatocellular carcinoma in
galectin-3 knockout mice. Hepatology Research. 38:1241–1251.
2008.PubMed/NCBI
|
|
15
|
Zucmann-Rossi J, Jeannot E, Nhieu JT,
Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis
V, Michalak S, et al: Genotype-phenotype correlation in
hepatocellular carcinoma: New classification and relationship with
HCC. Hepatology. 43:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zucmann-Rossi J, Benhamouche S, Godard C,
Boyault S, Grimber G, Balabaud C, Cunha AS, Bioulac-Sage P and
Perret C: Differential effects of inactivated Axin1 and activated
beta-catenin mutations in human hepatocellular carcinomas.
Oncogene. 26:774–780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bioulac-Sage P, Blanc JF, Rebouissou S,
Balabaud C and Zucmann-Rossi J: Genotype phenotype classification
of hepatocellular adenoma. World J Gastroenterol. 13:2649–2654.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akiyama TE, Ward JM and Gonzalez FJ:
Regulation of the liver fatty acid-binding protein gene by
hepatocyte nuclear factor 1alpha (HNF1alpha). Alterations in fatty
acid homeostasis in HNF1alpha-deficient mice. J Biol Chem.
275:27117–27122. 2000.PubMed/NCBI
|
|
19
|
Atshaves BP, Martin GG, Hostetler HA,
McIntosh AL, Kier AB and Schroeder F: Liver fatty acid-binding
protein and obesity. J Nutr Biochem. 21:1015–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inoue M, Takahashi Y, Fujii T, Kitagawa M
and Fukusato T: Significance of downregulation of liver fatty
acid-binding protein in hepatocellular carcinoma. World J
Gastroenterol. 20:17541–17551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alpini G, Elias I, Glaser SS, Rodgers RE,
Phinizy JL, Robertson WE, Francis H, Lasater J, Richards M and
LeSage GD: Gamma-Interferon inhibits secretin-induced choleresis
and cholangiocyte proliferation in a murine model of cirrhosis. J
Hepatol. 27:371–380. 1997. View Article : Google Scholar : PubMed/NCBI
|