Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer and the second most common cause of
cancer-related mortality worldwide (1). Curative resection or liver
transplantation is recommended for early-stage HCC, with a reported
5-year survival of >50% (2).
However, a considerable proportion of patients may develop HCC
recurrence and the survival of such patients is very poor, as
recurrent tumors are usually aggressive and unresectable (3). Moreover, HCC is significantly resistant
to radio- or chemotherapy, the standard of care in the majority of
advanced tumors (2).
Although the multikinase inhibitor sorafenib was
approved for the treatment of advanced HCC in 2008, there remain
issues regarding the management of this disease (4,5). In
particular, this therapy exhibits wide variability in terms of
prolongation of patient survival, and only few patients truly
benefit from this therapy. Combination therapy with sorafenib and
other modalities, such as transarterial chemoembolization or
everolimus, has been investigated, with some benefits (6–8).
A novel vaccine strategy for producing DRibbles,
whose major structure and functional constituents are
autophagosomes, has been developed (9). DRibbles has been found to be
significantly efficient in stimulating human antigen-specific CD8
memory T cells ex vivo and DRibbles-pulsed dendritic cell
(DC) immunization may induce T-cell response against HCC and result
in significant inhibition of tumor growth in mice (10,11). We
herein report the case of a patient with lung metastasis from HCC
following hepatectomy, who was treated with combination therapy
with sorafenib, focused ultrasound knife and DC-DRibbles vaccine
and achieved complete response.
Case report
A 42-year old male patient, who was diagnosed with
primary HCC associated with hepatitis B virus infection, underwent
curative hepatectomy in February, 2012. Enhanced computed
tomography (CT) findings revealed an intrahepatic giant HCC in the
right lobe of the liver, which was 100 mm in diameter. No
preoperative biopsy of the liver mass was conducted and HCC was
diagnosed according to radiological criteria (12). At the time of the diagnosis, the
patient presented with a good liver function reserve
(Child-Turcotte-Pugh class A). The α-fetoprotein (AFP) serum level
was 18,408.27 ng/ml (normal level, 10 ng/ml). Based on the
Barcelona Clinical Liver Cancer (BCLC) staging system, the tumor
was BCLC stage B, and the Eastern Cooperative Oncology Group (ECOG)
performance status score was 1 according to the general assessment.
The primary tumor was removed and it was found to be a G2-G3 HCC,
without hepatic portal lymphatic metastasis. In addition, no portal
vein thrombosis was detected.
Less than 2 months after surgery, recurrence of
multiple, bilateral liver lesions was observed on CT, of which the
largest was 85×69 mm in size, while simultaneous multiple pulmonary
metastases were observed in the lower part of the left lung. In
accordance with the recurrence of HCC, the AFP level increased to
37,202.46 ng/ml. In April, 2012, considering the presence of intra-
and extrahepatic metastases, the patient was placed on sorafenib
(Nexavar, Bayer) at a dosage of 400 mg b.i.d.; grade 1 hand-foot
syndrome developed. One month after the administration of
sorafenib, the AFP level increased to 103,295 ng/ml; thus, focused
ultrasound knife therapy for the liver lesions was repeated 7
times. At the same time, DC-DRibbles biotherapy was administered as
follows: The patient underwent apheresis to obtain peripheral blood
mononuclear cells (PBMCs), which were cultured for 3–5 days with
granulocyte-macrophage colony-stimulating factor (GM-CSF) and
interferon (IFN)-α to obtain IFN-DC on day 0 (13). IFN-DCs were administered by
subcutaneous injection (107 cells each time) on days 4,
18 and 31. HCC cell line SMMC-7721-derived DRibbles was prepared as
previously described (14). Briefly,
SMMC-7721 cells were treated with bortezomib (200 nmol/l) and
ammonium chloride (10–20 mmol/l) for 24–48 h. DRibbles was
dislodged from cells or large cell debris by rigorous pipetting.
The suspension was then centrifuged at 10,000 × g to harvest
DRibbles. The total amount of protein in DRibbles was quantified by
a bicinchoninic acid protein assay kit according to the
manufacturer's protocol (Thermo Scientific, Rockford, IL, USA). The
SMMC-7721 derived DRibbles (250 µg each time) was administered to
the inguinal lymph nodes (LNs) bilaterally under ultrasound
guidance, for a total of three times, separated by 2-week
intervals.
In June, 2012, a boost immunization of DC-DRibbles
vaccine was performed. DC was cultured in the same way as in the
first cycle and was loaded with DRibbles in vitro,
DC-DRibbles (107 cells each time) were administered
subcutaneously in the area of the inguinal LNs bilaterally, for a
total of 3 times, with 3-day intervals. On clinical evaluation, no
short-term or long-term toxicity was observed and the patient
exhibited adequate liver function and no signs of portal
hypertension throughout the entire duration of the follow-up.
In December, 2012, 8 months post-combination
therapy, the AFP level returned to normal, the patient had normal
liver function (Child-Pugh class A) and an ECOG score of 0.
Surprisingly, the CT scan revealed remission of the liver
metastases and disappearance of the lung metastases, which was a
significant improvement compared with the scan in April, 2012
(Fig. 1A1 and A2). The liver
metastases had disappear completely on the CT scan in June, 2015
(Fig. 1B1 and B2).
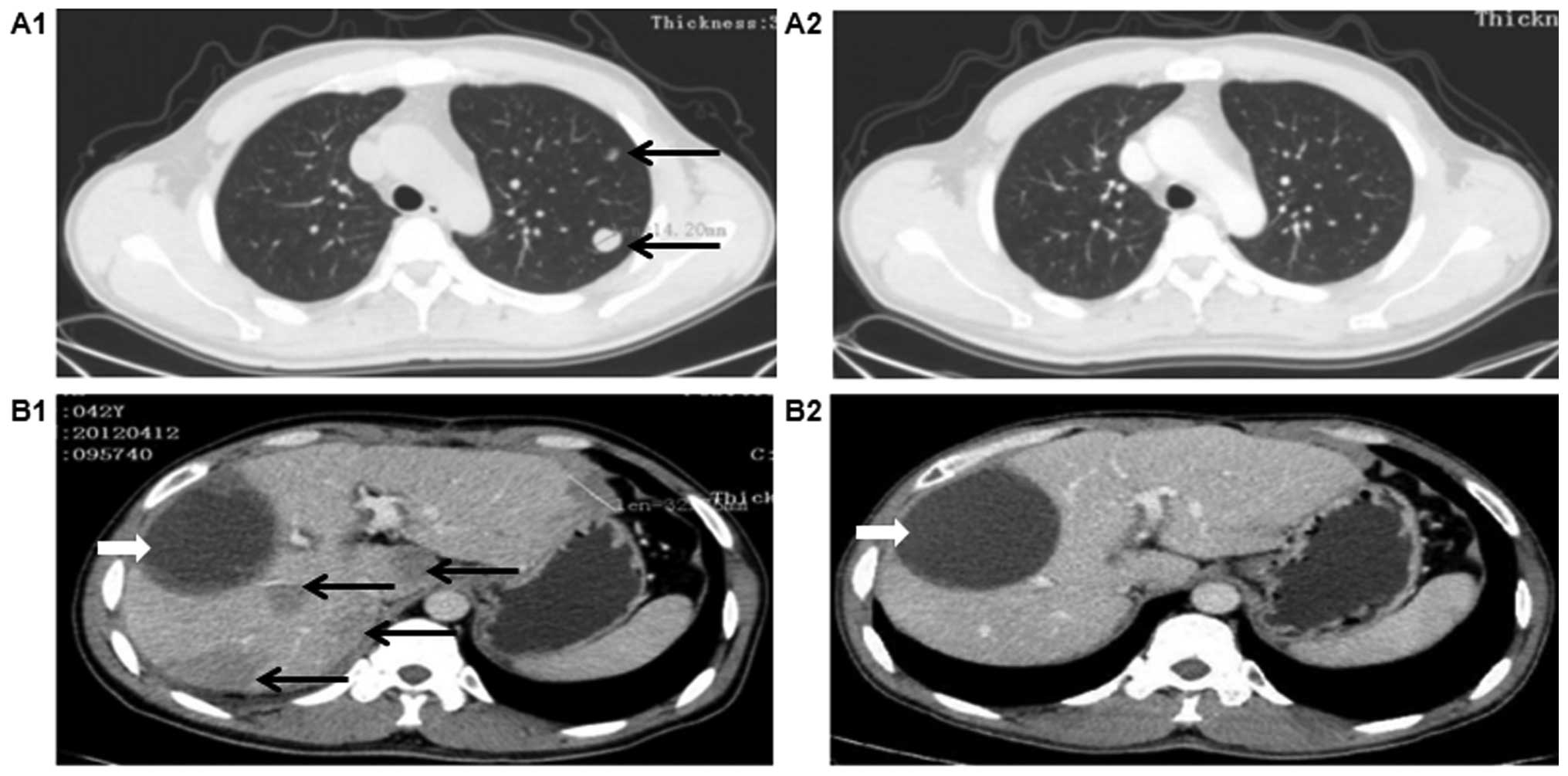 | Figure 1.Computed tomography response to
combination treatment with DC-DRibbles vaccine, sorafenib and
focused ultrasound knife. Prior to combination treatment, on April,
2012, (A1) multiple pulmonary metastases were identified (arrows)
and (B1) an intrahepatic giant hepatocellular carcinoma (HCC), with
multifocal, bilobar HCC recurrence following curative hepatectomy
found in the liver (black arrows, recurrence of HCC; white arrow,
change after hepatectomy). Following combination treatment, a
complete response was observed, with (A2) disappearance of lung
metastases by December, 2012 and (B2) disappearance of liver
metastases by June, 2015. |
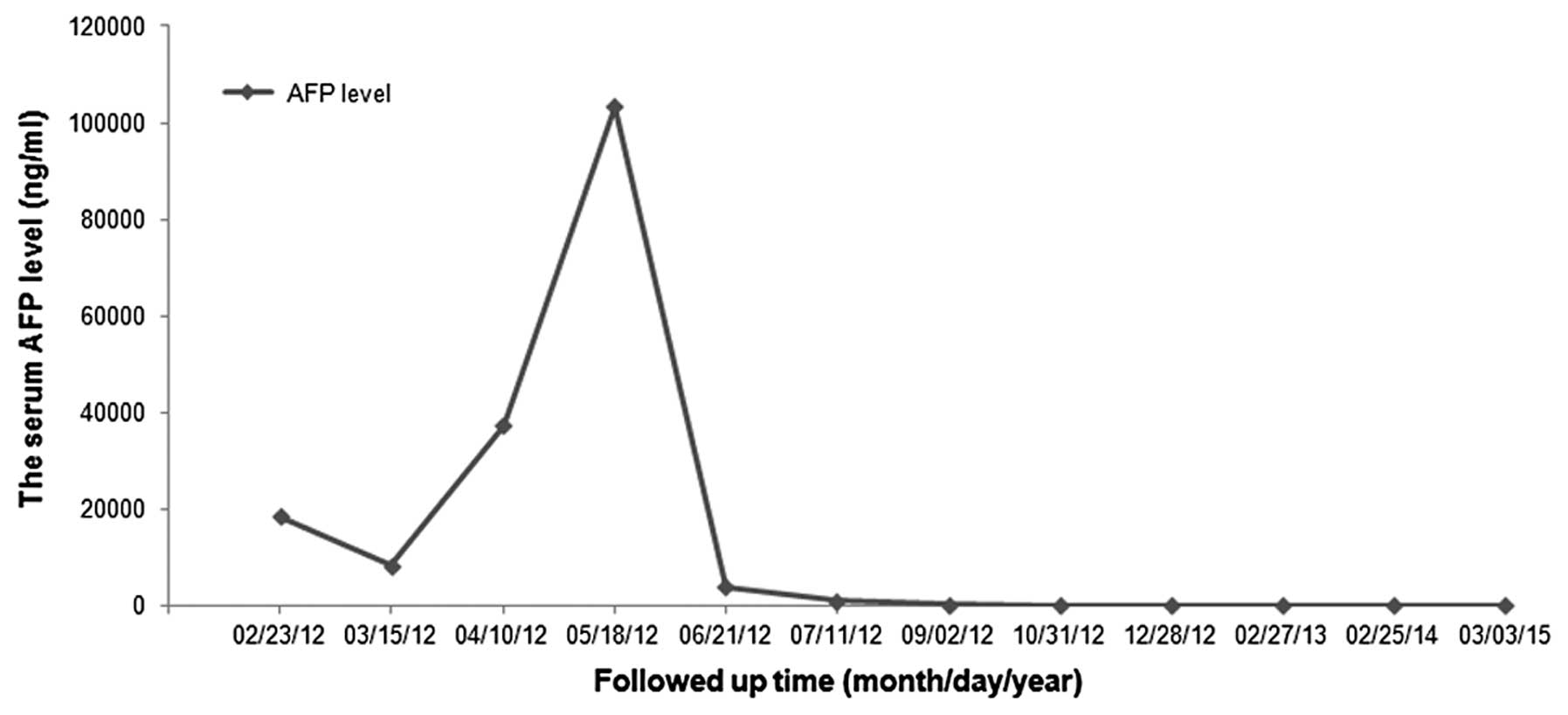
As a traditional indicator for evaluating the
curative effect and recurrence of HCC, the level of serum AFP was
measured every 1–2 months (Fig. 2)
and was found to be transiently decreased after the initial
surgical treatment, but swiftly increased to 37,202.46 ng/ml upon
recurrence of HCC, as demonstrated by CT scan. Following combined
treatment with DC-DRibbles vaccine, sorafenib and focused
ultrasound knife, the level of serum AFP decreased significantly
and returned to normal.
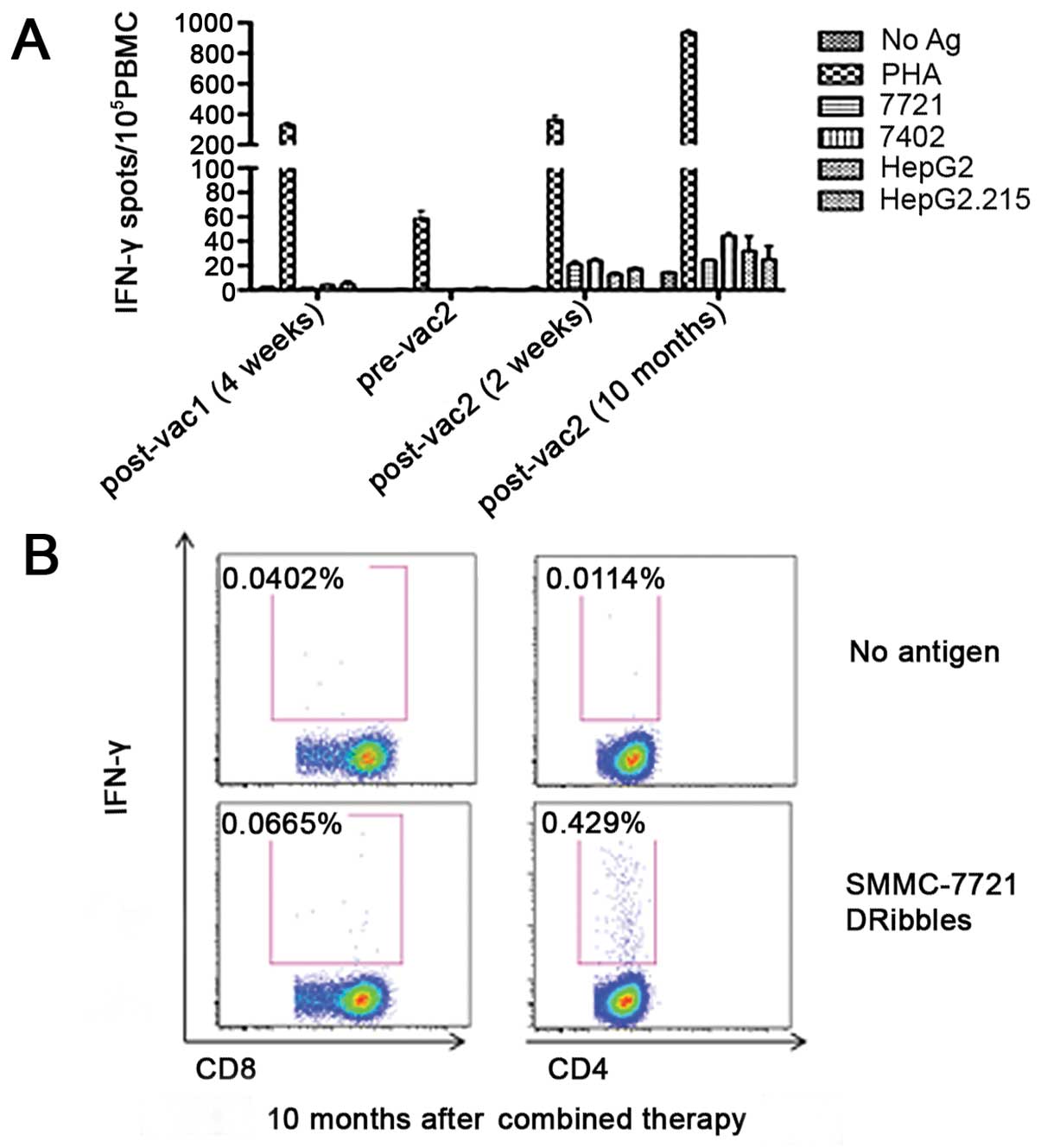
To evaluate the patient's specific T-cell response
to SMMC-7721-derived DRibbles vaccine, ELISPOT and intracellular
staining (ICS) analysis were performed to detect the secretion of
IFN-γ by T cells at different timepoints, as shown in Fig. 3. Briefly, PBMCs were isolated from
fresh blood by density gradient centrifugation in Ficoll solution
and seeded into a 96-well plate. DRibbles derived from different
liver tumor cell lines (SMMC-7721, BEL-7402, HepG2 and HepG2.215)
were pulsed with PBMCs to restimulate T cells at the concentration
of 25 µg/ml; the no antigen group served as negative control and
the phytohemagglutinin group as positive control. After
stimulation, ELISPOT and ICS analysis were conducted according to
the standard protocol. The ELISPOT data showed a low
antigen-specific T-cell response 4 weeks after the first vaccine
cycle and the response decreased to nearly zero prior to the second
cycle. However, high antigen-specific T-cell response was observed
2 weeks after the second vaccine cycle and did not decrease even
after 10 months, which was consistent with the result of the ICS
analysis. In addition, the ICS results demonstrated that most of
the secretory of IFN-γ was produced by CD4+ T cells,
whereas a low CD8+ T-cell response was observed (0.429
vs. 0.0665%, respectively).
Eight months after combined treatment with
DC-DRibbles vaccine, sorafenib and focused ultrasound knife, a
response of 87% according to the modified Response Evaluation
Criteria in Solid Tumors (mRECIST) (15) was observed on CT, with a change in
total diameter of contrast-enhanced areas in targeted tumor nodules
from 311.81 cm (in 12 nodules) to 29.37 cm (in 2 nodules). Three
years after the combined treatment, the tumor nodules had
disappeared completely in the lung and liver, according to the CT
scan.
Three years after the diagnosis of metastases and
post-combined therapy, the AFP serum level remains within the
normal range. Additionally, the enhanced CT scan has not revealed
any new recurrence and the patient remains Child-Pugh A and ECOG 0.
Treatment with sorafenib has been continued and a follow-up program
to evaluate the duration of the response of this combination
therapy is in progress. The patient also maintains a relatively
active lifestyle and no additional adverse events have been
reported.
Written informed consent was obtained from the
patient in accordance with the Declaration of Helsinki. The
treatment protocol was approved by the Human Research Ethics
Committee of the Second Affiliated Hospital of Southeast
University.
Discussion
The main therapeutic option for advanced-stage HCC
is sorafenib, an oral inhibitor of the vascular endothelial growth
factor receptor, the platelet-derived growth factor receptor, and
Raf. Sorafenib has been proven to prolong the survival of patients
with advanced HCC over placebo in the SHARP and the Asian-Pacific
pivotal studies (4,5). However, only a small proportion of
patients truly benefited from sorafenib treatment, and an even
smaller percentage of treated patients achieves partial or complete
response according to the RECIST criteria on follow-up
examinations. Thus, novel therapeutic strategies containing
sorafenib are urgently needed.
Autophagy is a basic cellular mechanism of degrading
cytoplasmic proteins and organelles and functions as a homeostatic
process in eukaryotic cells (16).
Autophagosomes are double-layered membrane structures produced in
the process of the sequestration of cytoplasmic components. Our
studies demonstrated that antigens sequestered in the autophagosome
may be delivered to DCs for cross-presentation and prime naïve
antigen-specific CD8 T cells effectively (17). Subsequently, autophagosomes containing
a broad spectrum of cellular antigens from antigen donor cells were
collected by induction of autophagy and inhibition of
lysosomal/proteosomal activity, and named DRibbles (9,14,18,19).
DRibbles have been demonstrated to exhibit vigorous antitumor
efficacy in mouse experiments and cross-prime human CD8 T cells in
ex vivo studies (10,11). In this study, we extracted DRibbles
from the HCC cell line SMMC7721 and injected them into the inguinal
LNs bilaterally in HCC patients. The results of flow cytometry, ICS
and ELISPOT demonstrated that tumor antigen-specific CD4 and CD8 T
cells could be primed in the human body. As DRibbles were used as
stimulating antigens in the detection, the exact antigen-specific
T-cell epitopes could not be identified.
Cross-presentation is crucial for the activation of
CD8 T cells primed by exogenous antigens (20). DCs are the most potent professional
antigen-presenting cells (pAPCs) in humans. In this study, LN DCs
and monocyte-derived DCs were used as pAPCs to cross-present
DRibbles antigens. In vivo cross-presentation occurs in
secondary lymphoid organs, particularly LNs containing mature
CD1c+ conventional DCs (cDCs), CD141+ cDCs,
and plasmacytoid DCs (21).
SMMC7721-DRibbles was injected directly into the inguinal LNs
bilaterally. We hypothesize that LN DC subsets may internalize and
degrade DRibbles and display the resulting peptides on their cell
surface during the process of cross-presentation. Recently, CLEC9A
was shown to recognize dead cell-associated antigen and play a
critical role in cross-presentation of antigens from necrotic cells
(22,23). DRibbles was found to express abundant
CLEC9A ligand and may directly bind to CLEC9A expressed on the
surface of CD141+ cDC subsets. This suggests that the
CD141+ cDC subset may be involved in the recognition or
subsequent procession of DRibbles by DCs (14). In the presence of GM-CSF and IFN-α,
monocytes may differentiate into IFN-DCs in vitro, which may
have a more potent function in terms of CD8 T-cell cross-priming
compared with traditional interleukin (IL)-4-DCs generated from
monocytes stimulated by GM-CSF and IL-4 (13,24). It
was previously demonstrated that IFN-DCs may cross-present DRibbles
and prime human memory antigen-specific CD8 and CD4 T cells more
effectively compared with IL-4-DCs (data not shown). Consequently,
the IFN-DC-DRibbles vaccine strategy was also implemented in our
patient. The results demonstrated that antigen-specific CD8 and CD4
T cells may be activated, but we were unable to discriminate the
type of DCs playing the major role between LN DCs and IFN-DCs.
In the present case, the sorafenib-DC-DRibbles
combination led to complete tumor response, whereas no serious side
effects were observed. We hypothesize that there is a synergistic
action of these two therapeutic strategies, as the exact mechanism
could not be illustrated exactly. The success of the treatment in
the present case supports the value of combined strategy of
targeted therapy and immunological therapy in patients with
recurrent HCC.
Acknowledgements
The present study was supported by grants from the
Nanjing Medical Science and Technique Development Foundation,
Nanjing Department of Health (nos. QRX11235 and ZDX12008) and the
Jiangsu Provincial Clinical Medical Science and Technology project
(no. BL2014005).
References
|
1
|
Au JS and Frenette CT: Management of
hepatocellular carcinoma: Current status and future directions. Gut
Liver. 9:437–448. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colagrande S, Regini F, Taliani GG, Nardi
C and Inghilesi AL: Advanced hepatocellular carcinoma and
sorafenib: Diagnosis, indications, clinical and radiological
follow-up. World J Hepatol. 7:1041–1053. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeishi K, Maeda T, Tsujita E, Yamashita
Y, Harada N, Itoh S, Harimoto N, Ikegami T, Yoshizumi T, Shirabe K
and Maehara Y: Predictors of intrahepatic multiple recurrences
after curative hepatectomy for hepatocellular carcinoma. Anticancer
Res. 35:3061–3066. 2015.PubMed/NCBI
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR,
Bhagat N and Geschwind JF: Phase II trial of sorafenib combined
with concurrent transarterial chemoembolization with drug-eluting
beads for hepatocellular carcinoma. J Clin Oncol. 29:3960–3967.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han G, Yang J, Shao G, Teng G, Wang M,
Yang J, Liu Z, Feng G, Yang R, Lu L, et al: Sorafenib in
combination with transarterial chemoembolization in Chinese
patients with hepatocellular carcinoma: A subgroup interim analysis
of the START trial. Future Oncol. 9:403–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhoori S, Toffanin S, Sposito C, Germini
A, Pellegrinelli A, Lampis A and Mazzaferro V: Personalized
molecular targeted therapy in advanced, recurrent hepatocellular
carcinoma after liver transplantation: A proof of principle. J
Hepatol. 52:771–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi Y, Zhou Z, Shu S, Fang Y, Twitty C,
Hilton TL, Aung S, Urba WJ, Fox BA, Hu HM and Li Y:
Autophagy-assisted antigen cross-presentation: Autophagosome as the
argo of shared tumor-specific antigens and DAMPs. Oncoimmunology.
1:976–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye W, Xing Y, Paustian C, van de Ven R,
Moudgil T, Hilton TL, Fox BA, Urba WJ, Zhao W and Hu HM:
Cross-presentation of viral antigens in dribbles leads to efficient
activation of virus-specific human memory T cells. J Transl Med.
12:1002014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su S, Zhou H, Xue M, Liu JY, Ding L, Cao
M, Zhou ZX, Hu HM and Wang LX: Anti-tumor efficacy of a
hepatocellular carcinoma vaccine based on dendritic cells combined
with tumor-derived autophagosomes in murine models. Asian Pac J
Cancer Prev. 14:3109–3116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lapenta C, Santini SM, Spada M, Donati S,
Urbani F, Accapezzato D, Franceschini D, Andreotti M, Barnaba V and
Belardelli F: IFN-alpha-conditioned dendritic cells are highly
efficient in inducing cross-priming CD8+ T cells against
exogenous viral antigens. Eur J Immunol. 36:2046–2060. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Wang LX, Pang P, Cui Z, Aung S,
Haley D, Fox BA, Urba WJ and Hu HM: Tumor-derived autophagosome
vaccine: Mechanism of cross-presentation and therapeutic efficacy.
Clin Cancer Res. 17:7047–7057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D and
Verweij J: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:1845–1846. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Wang LX, Yang G, Hao F, Urba WJ and
Hu HM: Efficient cross-presentation depends on autophagy in tumor
cells. Cancer Res. 68:6889–6895. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Hahn T, Garrison K, Cui ZH, Thorburn
A, Thorburn J, Hu HM and Akporiaye ET: The vitamin E analogue α-TEA
stimulates tumor autophagy and enhances antigen cross-presentation.
Cancer Res. 72:3535–3545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Twitty CG, Jensen SM, Hu HM and Fox BA:
Tumor-derived autophagosome vaccine: Induction of cross-protective
immune responses against short-lived proteins through a
p62-dependent mechanism. Clin Cancer Res. 17:6467–6481. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Segura E and Amigorena S:
Cross-presentation by human dendritic cell subsets. Immunol Lett.
158:73–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boltjes A and van Wijk F: Human dendritic
cell functional specialization in steady-state and inflammation.
Front Immunol. 5:1312014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schreibelt G, Klinkenberg LJ, Cruz LJ,
Tacken PJ, Tel J, Kreutz M, Adema GJ, Brown GD, Figdor CG and de
Vries IJ: The C-type lectin receptor CLEC9A mediates antigen uptake
and (cross-)presentation by human blood BDCA3+ myeloid
dendritic cells. Blood. 119:2284–2292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poulin LF, Salio M, Griessinger E,
Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S,
Nye E, et al: Characterization of human DNGR-1+
BDCA3+ leukocytes as putative equivalents of mouse
CD8alpha+ dendritic cells. J Exp Med. 207:1261–1271.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ng D and Gommerman JL: The Regulation of
Immune Responses by DC Derived Type I IFN. Front Immunol. 4:942013.
View Article : Google Scholar : PubMed/NCBI
|