Introduction
Oxygen is essential for aerobic organisms and a lack
of oxygen results in considerable stresses to these organisms.
Cells have a variety of hypoxia response mechanisms to counter
hypoxic stress. The gene, hypoxia-inducible factor
(HIF)-1, serves an important role in many of these
mechanisms (1–7). The HIF-1 protein is a complex of HIF-1α
and HIF-1β. HIF-1β is present constitutively within the cell
nucleus. By contrast, HIF-1α is present in the cytoplasm under
normoxic conditions, and it promptly binds to the von Hippel-Lindau
(VHL) protein and is decomposed by the ubiquitin proteasome system.
In hypoxic conditions, HIF-1α is unable to bind to the VHL protein
in the cytoplasm, escapes decomposition and enters the nucleus. In
the nucleus, it combines with HIF-1β to form the HIF-1 complex,
which binds to DNA and acts as a transcription factor. HIF-1
assists in the activation of genes involved in angiogenesis,
glycolysis, tumor proliferation and other associated pathways
(1–7).
Solid cancers generally grow very rapidly and these
tumors often face a shortage of oxygen since tumor angiogenesis is
slower than growth. Therefore, numerous cancer types use the HIF-1
complex to adapt to hypoxia (1–3). A
previous study reported that HIF-1α expression was associated with
tumor proliferation, antiapoptosis, angiogenesis, glycolysis and
patient prognosis (4–7).
Few studies have examined HIF-1α expression in
surgically resected lung cancer (8,9).
Accordingly, the present study decided to perform a comprehensive
clinicopathological study of the intratumoral expression of HIF-1α
in resected lung cancers, investigating the associations between
HIF-1α expression, other tumor malignancy indicators [survivin
(10–15) and c-Myc (16–18)] and
the clinicopathological features of the patients.
Patients and methods
Patients
The present study included 216 consecutive patients
with lung cancer who underwent a pulmonary resection at Tokyo
Medical and Dental University Hospital (Tokyo, Japan) between April
2013 and January 2015. The histological tumor classification was
based on the 7th lung cancer tumor node metastasis
classification and the staging system proposed by the International
Union Against Cancer (19). The
clinical records and histological examination results of all
patients were fully documented. High-resolution computed tomography
was performed in all patients within 1 month prior to resection.
The ground glass opacity (GGO) ratio was calculated in a lung
window using the following formula: (1 - solid tumor size / GGO
size) ×100. Positron emission tomography was performed in 176
patients (82.2%) and the maximum standardized uptake value (SUVmax)
of the tumor was measured in these patients. Patients who received
chemotherapy or radiotherapy prior to resection were excluded. The
study was approved by the Ethics Committee of Tokyo Medical and
Dental University Hospital and informed consent was obtained from
each patient.
Immunohistochemical analysis
The antibodies used for immunohistochemical analysis
were mouse monoclonal anti-Ki-67 (MIB-1; cat. no. M7240; Dako
Denmark A/S, Glostrup, Denmark; 1:100), mouse monoclonal
anti-survivin (D-8; cat. no. sc-17779; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA; 1:50), mouse monoclonal anti-c-Myc
(9E10; cat. no. sc-40; Santa Cruz Biotechnology, Inc.; 1:100) and
mouse monoclonal anti-HIF-1α (H1 alpha 67; cat. no. NB100-105;
Novus Biologicals, Littleton, CO, USA; 1:50). Formalin-fixed
paraffin-embedded tissues were cut into 4 µm sections and mounted
onto coated slides. Following deparaffinization and rehydration,
the slides were heated in a microwave (10 min for Ki-67, 25 min for
survivin, 5 min for c-Myc and 30 min for HIF-1α) in a citrate
buffer solution (10 µmol/l) at pH 6.0. After quenching the
endogenous peroxidase activity with 0.3% H2O2
for 30 min, the sections were treated with 5% bovine serum albumin
(Sigma-Aldrich, St. Louis, MO, USA). Duplicate sections were
incubated overnight with the corresponding primary antibody. The
sections were subsequently incubated with the
avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame,
CA, USA) for 30 min and antibody binding was visualized with
3,3′-diaminobenzine tetrahydrochloride. The tissue sections were
counterstained with Mayer's hematoxylin.
The immunostained sections were examined by two
authors (Chihiro Takasaki and Masashi Kobayashi), in a blinded
manner. At least 200 tumor cells were scored per field under ×40
magnification. The percentages of tumor cells with positive
staining for Ki-67, survivin, c-Myc and HIF-1α were determined
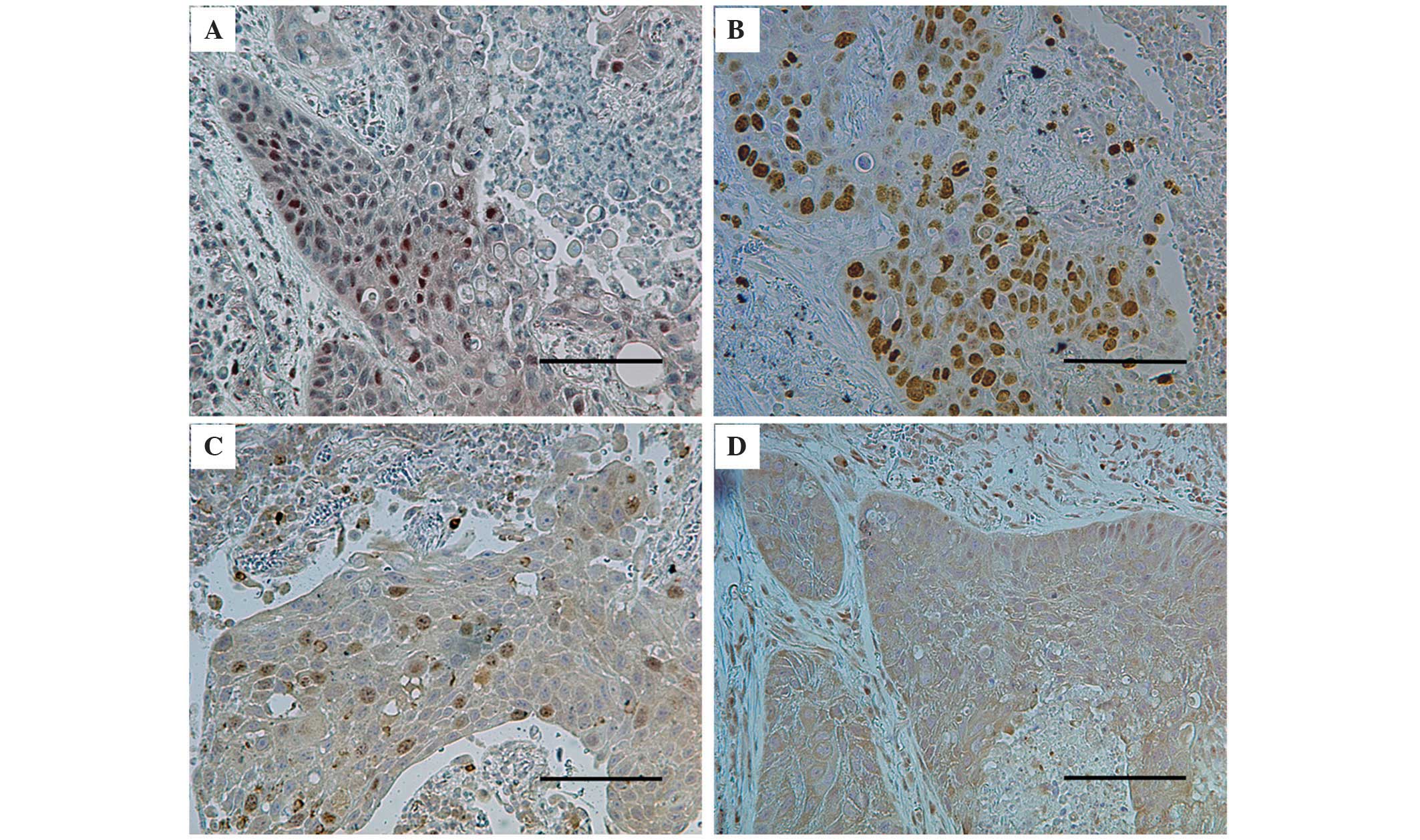
(Fig. 1). Discrepancies were jointly
re-evaluated until a consensus was reached.
Statistical analysis
The statistical analysis was performed using
StatView-J Software (version 5.0; SAS Institute Inc., Cary, NC,
USA). Based on previous reports (8,9,20), all samples were divided into two
groups: A high-HIF-1α tumor group, in which the percentage of
HIF-1α-positive cells was ≥20%, and a low-HIF-1α tumor group, in
which the percentage of HIF-1α-positive cell was <20%. The
χ2-test was used to assess the association between
HIF-1α expression and categorical variables, including patient
gender, smoking habits, pathological tumor stage, histological
tumor type, vascular invasion, tumor differentiation and SUVmax
(The tumor sample was considered to have a high SUVmax if the
SUVmax was >5.0.). The t-test was used to assess the association
between HIF-1α expression and continuous variables, including
patient age, tumor size, GGO ratio, Ki-67 proliferation index,
survivin expression and c-Myc expression. All continuous variables
were summarized in terms of their means and standard deviations.
When investigating the association between the Ki-67 proliferation
index and the survivin and c-Myc expression in the tumors, the
sample was divided into two groups according to the rates of
survivin and c-Myc expression, as follows: The sample was
classified as a nuclear survivin-high tumor if the percentage of
nuclear survivin-positive cells was ≥17% and the tumor was
classified as a c-Myc-high tumor if the percentage of
c-Myc-positive cells was ≥40%. These thresholds were selected as
they had the highest significance values in relation to the Ki-67
proliferation index (21).
A multivariate analysis using a logistic regression
model was performed to determine the independent factors for high
HIF-1α expression. All statistical tests were two-tailed and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The characteristics of the patients are presented in
Table I. The study included 216
patients with resected lung cancer, of whom 54 had squamous cell
carcinoma (25.0%) and 162 had non-squamous cell carcinoma (75%),
including adenocarcinoma, adenosquamous cell carcinoma, large cell
carcinoma, large cell neuroendocrine carcinoma, and small cell
carcinoma. With respect to tumor differentiation, 148 patients had
≥G2-grade disease and 68 patients had G1-grade disease.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | No. patients | (%) |
|---|
| Total | 216 | 100.0 |
| Age, years |
|
|
|
Median | 72 | − |
|
Range | 39–89 | − |
| Gender |
|
|
| Male | 138 | 63.9 |
|
Female | 78 | 36.1 |
| Smoking status |
|
|
|
Non-smoker (BI ≤100) | 53 | 24.5 |
|
Smoker | 163 | 75.5 |
| Pathological tumor
stage |
|
|
| IA | 92 | 42.6 |
| IB | 55 | 25.5 |
| IIA | 17 | 7.9 |
| IIB | 14 | 6.5 |
| IIIA | 36 | 16.6 |
| IIIB | 0 | 0.0 |
| IV | 2 | 1.0 |
| Histological tumor
type |
|
|
|
Squamous | 54 | 25.0 |
|
Non-squamous | 162 | 75.0 |
| Lymphatic
invasion |
|
|
| + | 38 | 17.6 |
| − | 178 | 82.4 |
| Venous invasion |
|
|
| + | 108 | 50.0 |
| − | 108 | 50.0 |
| Tumor size (mm) |
|
|
|
Median | 31 | − |
|
Range | 9–82 | − |
| GGO ratio (%) |
|
|
|
Median | 16.3 | − |
|
Range | 0–100 | − |
| SUVmax |
|
|
|
Median | 4.4 | − |
|
Range | 0–27 | − |
| Tumor
differentiation |
|
|
| G1 | 58 | 28.2 |
|
≥G2 | 148 | 71.8 |
Clinical significance of HIF-1α
expression
The immunohistochemical staining for HIF-1α revealed
a nuclear pattern (Fig. 1). Among the
216 tumors, the mean percentage of HIF-1α-positive cells was
14.6±24.3%. Based on the 20% threshold for high HIF-1α, 58 (26.9%)
high-HIF-1α tumors and 158 (73.1%) low-HIF-1α tumors were
observed.
Regarding the patients' clinical characteristics, no
significant associations between patient age, gender, smoking
index, and HIF-1α expression were observed. With respect to the
preoperative imaging findings, the mean GGO ratios were 8.9±21.0%
for high-HIF-1α tumors and 19.0±30.0% for low-HIF-1α tumors; the
GGO ratio was significantly lower for high-HIF-1α tumors compared
with for low-HIF-1α tumors (P=0.02; Table II). The rate of tumors with high
SUVmax was significantly greater in the high-HIF-1α tumor group
compared with that in the low-HIF-1α tumor group (P<0.01;
Table II).
 | Table II.Association between HIF-1α expression
and the clinicopathological characteristics. |
Table II.
Association between HIF-1α expression
and the clinicopathological characteristics.
|
| HIF-1α
expression |
|
|---|
|
|
|
|
|---|
| Characteristic | High | Low | P-value |
|---|
| Mean age
(years) | 70.8 | 70.0 | 0.56 |
| Gender (n) |
|
|
|
|
Male | 39 | 99 | 0.64 |
|
Female | 19 | 59 |
|
| Smoking status
(n) |
|
|
|
|
Non-smoker | 12 | 41 | 0.54 |
|
Smoker | 46 | 117 |
|
| Pathological tumor
stage (n) |
|
|
|
| I | 38 | 109 | 0.75 |
|
≥II | 20 | 49 |
|
| Histological tumor
type (n) |
|
|
|
|
Squamous | 27 | 27 | <0.01 |
|
Non-squamous | 31 | 131 |
|
| Lymphatic invasion
(n) |
|
|
|
| + | 16 | 22 | 0.03 |
| − | 42 | 136 |
|
| Venous invasion
(n) |
|
|
|
| + | 35 | 73 | 0.09 |
| − | 23 | 85 |
|
| Mean tumor size
(mm) | 33.4 | 30.2 | 0.18 |
| Mean GGO ratio
(%) | 8.9 | 19.0 | 0.02 |
| SUVmax (n) |
|
|
|
| ≥5 | 30 | 50 | <0.01 |
|
<5 | 17 | 79 |
|
| Tumor
differentiation (n) |
|
|
|
| G1 | 9 | 49 | 0.03 |
|
≥G2 | 47 | 101 |
|
Regarding the tumors' pathological features, the
rate of squamous cell carcinoma was significantly higher in the
high-HIF-1α tumors compared with that in the low-HIF-1α tumors
(P<0.01; Table II). The lymphatic
invasion rate was also significantly higher in the high-HIF-1α
tumors compared with in low-HIF-1α tumors (P=0.03; Table II). With respect to tumor
differentiation, low-grade (≥G2) tumors had a significant
association with high-HIF-1α tumors. No association was observed
between pathological stage and HIF-1α expression.
The multivariate logistic regression analysis
identified squamous cell carcinoma (P=0.0009), lymphatic invasion
(P=0.0350), and high SUVmax (P=0.0471) as significant and
independent factors for high HIF-1α expression in patients with
lung cancer.
Associations between the expression
levels of HIF-1α, survivin and c-Myc, and the Ki-67 proliferation
index
Immunohistochemical staining for survivin revealed a
nuclear and/or cytoplasmic pattern (Fig.
1). The mean percentage of nuclear survivin-positive tumor
cells was 13.7±20.3% and that of cytoplasmic survivin-positive
tumor cells was 31.8±36.9%. The nuclear survivin expression was
significantly higher in the high-HIF-1α tumors compared with in the
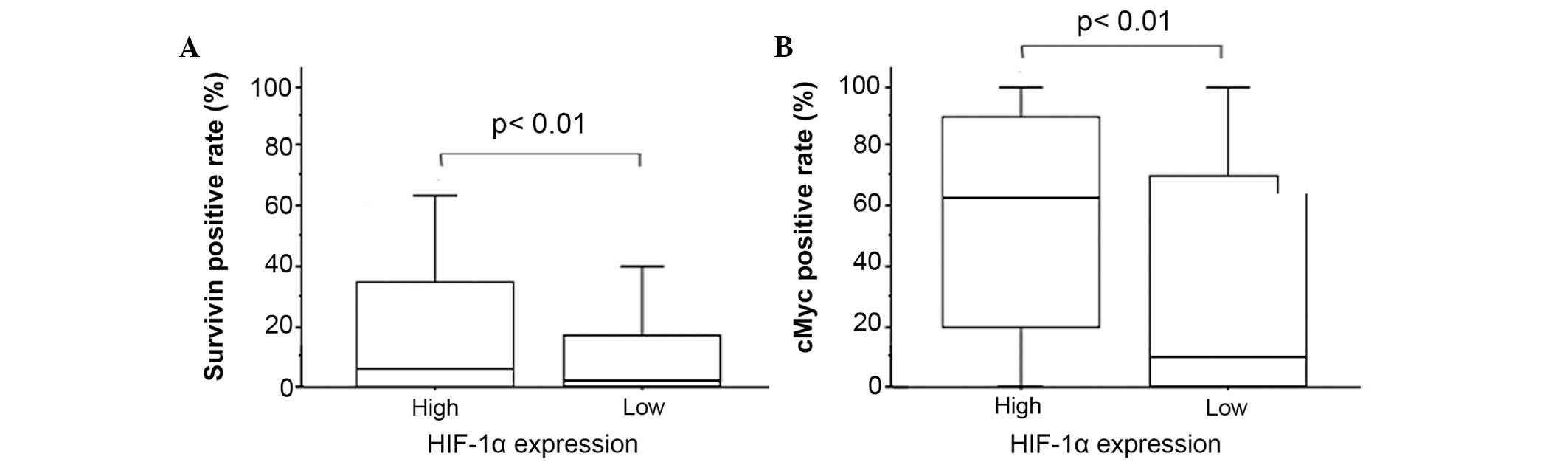
low-HIF-1α tumors (20.3±25.3 vs. 11.2±17.7%; P<0.01; Table III; Fig.
2). No difference in HIF-1α expression was observed according
to cytoplasmic survivin.
 | Table III.Association between HIF-1α and other
immunohistochemical markers. |
Table III.
Association between HIF-1α and other
immunohistochemical markers.
|
| HIF-1α
expression |
|
|---|
|
|
|
|
|---|
| Tumor marker
(%) | High | Low | P-value |
|---|
| Ki-67 | 47.5 | 34.6 |
0.01 |
| Survivin | 20.3 | 11.2 | <0.01 |
| c-Myc | 54.3 | 34.5 | <0.01 |
Immunohistochemical staining for c-Myc revealed a
cytoplasmic pattern following staining (Fig. 1). The mean percentage of
c-Myc-positive tumor cells was 39.8±38.8% among all included
tumors. c-Myc expression was significantly higher in the
high-HIF-1α tumors compared with the low-HIF-1α tumors (54.3±37.1
vs. 34.5±38.2%; P<0.01; Table
III; Fig. 2).
With regards to Ki-67, the Ki-67 proliferation index
was significantly higher in the high-HIF-1α tumor group compared
with that in the low-HIF-1α tumor group (47.5±30.9 vs. 34.6±32.9%;
P=0.01; Table III). The Ki-67
proliferation index also had significant associations with
high-survivin tumors and high-c-Myc tumors: The Ki-67-positive
rates for high- and low-survivin tumors were 58.1±28.8 and
29.7±30.8%, respectively (P<0.01), while the Ki-67-positive
rates for high- and low-c-Myc tumors were 47.0±31.1 and 30.1±32.3%,
respectively (P<0.01).
Discussion
A variety of genes are involved in tumor
proliferation, invasion and metastasis. HIF-1α is a transcriptional
factor that controls situations, including angiogenesis, glucose
metabolism and antiapoptosis. HIF-1α is expected to be an
important gene that cancers utilize during growth (4–7).
Survivin and c-Myc are genes that are known to be
highly expressed in certain cancer types. Survivin is a member of
the inhibitor of apoptosis protein family and it inhibits the
activation of caspase included in the apoptotic pathway (10,13–15). c-Myc
is a famous transcriptional factor that has been observed in
various types of cancers. It serves a role in cell-cycle
progression through the stimulation and repression of the
expression of cell-cycle regulators (17,18,22,23).
Although the signaling pathways between HIF-1α, survivin and c-Myc
remain to be sufficiently elucidated, certain previous reports have
noted that HIF-1α is a regulator of survivin and c-Myc, and also
acts as their transcription factor (15,23).
The present study revealed that squamous cell
carcinoma, a low GGO ratio and a high SUVmax were significantly
associated with HIF-1α expression. These findings indicated that
the solid tumors were sometimes subjected to low-oxygen conditions
and expressed HIF-1α to adapt to hypoxia. The pathological features
of the patients also showed that positive lymphatic invasion and
high tumor differentiation were correlated with HIF-1α expression,
indicating an association between HIF-1α and tumor proliferation.
Regarding the immunohistochemistry results, the present study
revealed that HIF-1α expression was significantly associated with
the expression levels of survivin and c-Myc, indicating that HIF-1α
was associated with tumor proliferation and antiapoptosis in
resected lung cancer. The observed correlation between HIF-1α
expression and the Ki-67 proliferation index is also suggestive of
the association between HIF-1α and tumor proliferation.
Previous studies have identified HIF-1α expression
in various cancer types, and HIF-1α-targeted therapy has started to
be applied clinically to several cancers (24,25). For
example, the molecularly targeted drug, everolimus, has been
applied to renal cell cancer and pancreatic endocrine tumors.
Everolimus inhibits mammalian target of rapamycin (mTOR), a
HIF-1α translation activator, and reduces the quantity of
HIF-1α protein. Other HIF-1α-targeted drugs are in clinical trials,
including HIF-1α small interfering RNA. Furthermore, a drug that
inhibits the binding of the HIF complex to DNA (GL331, echinomycin)
has been included cell-line experiments (24,25).
Although no HIF-1α-targeted therapy that has been adapted for lung
cancer currently exists, these HIF-1α-targeted therapies are
expected to provide novel treatments for lung cancer in the future.
Appropriate patient selection will become a key issue when these
anti-HIF-1 treatments are clinically applied to lung cancer.
Subgroups of patients in which high HIF-1α expression is expected
may represent good candidates for these therapies, including
patients of squamous cell carcinoma with positive lymphatic
invasion, low tumor differentiation, a low GGO ratio and a high
SUVmax.
In conclusion, the present study demonstrated that
HIF-1α expression was associated with tumor proliferation and
antiapoptosis in lung cancer via the expression of survivin and
c-Myc. Future studies involving clinical HIF-1α-targeted therapy
are warranted.
Glossary
Abbreviations
Abbreviations:
|
GGO
|
ground glass opacity
|
|
HIF-1
|
hypoxia-inducible factor-1
|
|
VHL
|
von hippel-lindau
|
References
|
1
|
Semenza GL: Expression of
hypoxia-inducible factor 1: Mechanisms and consequences. Biochem
Pharmacol. 59:47–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thrash-Bingham CA and Tartof KD: aHIF: A
natural antisense transcript overexpressed in human renal cancer
and during hypoxia. J Natl Cancer Inst. 91:143–151. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harada H, Itasaka S, Kizaka-Kondoh S,
Shibuya K, Morinibu A, Shinomiya K and Hiraoka M: The Akt/mTOR
pathway assures the synthesis of HIF-1alpha protein in a glucose-
and reoxygenation-dependent manner in irradiated tumors. J Biol
Chem. 284:5332–5342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaelin WG Jr: The won Hippel-Lindau tumor
suppressor protein: O2 sensing and cancer. Nat Rev Cancer.
8:865–873. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simon F, Bockhorn M, Praha C, Baba HA,
Broelsch CE, Frilling A and Weber F: Deregulation of HIF1-alpha and
hypoxia-regulated pathways in hepatocellular carcinoma and
corresponding non-malignant liver tissue-influence of a modulated
host stroma on the prognosis of HCC. Langenbecks Arch Surg.
395:395–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giles RH, Lolkema MP, Snijckers CM,
Belderbos M, van der Groep P, Mans DA, van Beest M, van Noort M,
Goldschmeding R, van Diest PJ, et al: Interplay between
VHL/HIF1alpha and Wnt/beta-catenin pathways during colorectal
tumorigenesis. Oncogene. 25:3065–3070. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SJ, Rabbani ZN, Dewhirst MW,
Vujaskovic Z, Vollmer RT, Schreiber EG, Oosterwijk E and Kelley MJ:
Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically
resected non-small cell lung cancer. Lung Cancer. 49:325–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volm M and Koomägi R: Hypoxia-inducible
factor (HIF-1) and its relationship to apoptosis and proliferation
in lung cancer. Anticancer Res. 20:1527–1533. 2000.PubMed/NCBI
|
|
10
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu N, Sun Y, Zhao N and Chen L: Role of
hypoxia-inducible factor-1α and survivin in oxygen-induced
retinopathy in mice. Int J Clin Exp Pathol. 7:6814–6819.
2014.PubMed/NCBI
|
|
12
|
Wu XY, Fu ZX and Wang XH: Effect of
hypoxia-inducible factor 1-α on survivin in colorectal cancer. Mol
Med Rep. 3:409–415. 2010.PubMed/NCBI
|
|
13
|
Chen XQ, Zhao CL and Li W: Effect of
hypoxia-inducible factor-1alpha on transcription of survivin in
non-small cell lung cancer. J Exp Clin Cancer Res. 28:292009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shinohara ET, Gonzalez A, Massion PP, Chen
H, Li M, Freyer AS, Olson SJ, Andersen JJ, Shyr Y, Carbone DP, et
al: Nuclear survivin predicts recurrence and poor survival in
patients with resected nonsmall cell lung carcinoma. Cancer.
103:1685–1692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brennan DJ, Rexhepaj E, O'Brien SL,
McSherry E, O'Connor DP, Fagan A, Culhane AC, Higgins DG, Jirstrom
K, Millikan RC, et al: Altered cytoplasmic-to-nuclear ratio of
surivin is a prognostic indicator in breast cancer. Clin Cancer
Res. 14:2681–2689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koshiji M, Kageyama Y, Pete EA, Horikawa
I, Barret JC and Huang LE: HIF-1alpha induces cell cycle arrest by
functionally counteracting Myc. EMBO J. 23:1949–1956. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pelengaris S, Khan M and Evan G: c-Myc:
More than just a matter of life and death. Nat Rev Cancer.
2:764–776. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dang CV, Resar LM, Emison E, Kim S, Li Q,
Prescott JE, Wonsey D and Zeller K: Function of the c-Myc oncogenec
transcription factor. Exp Cell Res. 253:63–77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC lung cancer staging project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM classification of malignant tumors. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karetsi E, Ioannou MG, Kerenidi T, Minas
M, Molyvdas PA, Gourgoulianis KL and Paraskeva E: Differential
expression of hypoxia-inducible factor-1α in non-small cell lung
cancer and small cell lung cancer. Clinics (Sao Paulo).
67:1373–1378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kobayashi M, Hyang CL, Sonobe M, Kikuchi
R, Ishikawa M, Kitamura J, Miyahara R, Menju T, Iwakiri S, Itoi K,
et al: Intratumoral Wnt2B expression affects tumor proliferation
and survival in malignant pleural mesothelioma patients. Exp Ther
Med. 3:952–958. 2012.PubMed/NCBI
|
|
22
|
Dang CV, Kim JW, Gao P and Yustein J: The
interplay between MYC and HIF in cancer. Nat Rev Cancer. 8:51–56.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang LE: Carrot and stick: HIF-alpha
engages c-Myc in hypoxic adaptation. Cell Death Differ. 15:672–677.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Semenza GL: Evaluation of HIF-1 inhibitors
as anticancer agents. Drug Discov Today. 12:853–859. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujita M, Yasuda M, Kitatani K, Miyazawa
M, Hirabayashi K, Takekoshi S, Iida T, Hirasawa T, Murakami M,
Mikami M, et al: An up-to-date anti-cancer treatment strategy
focusing on HIF-1alpha suppression: Its application for refractory
ovarian cancer. Acta Histochem Cytochem. 40:139–142. 2007.
View Article : Google Scholar : PubMed/NCBI
|