Introduction
Pancreatic cancer has the worst prognosis among all
refractory gastrointestinal cancers. According to data on the
number of site-specific cancer deaths in Japan, pancreatic is the
fourth most common cancer, after lung, stomach and colon cancers
(1). In 1997, randomised clinical
trials comparing gemcitabine (GEM) and 5-fluorouracil (5-FU)
chemotherapy for pancreatic cancer (2), demonstrated that GEM was more beneficial
for symptom relief compared with 5-FU, and also prolonged survival.
In addition, since August, 2006, an oral 5-FU formulation
containing tegafur, gimeracil and oteracil potassium (S-1) has been
approved by insurance companies for the treatment of pancreatic
cancer. The GEM and S-1 trial (GEST study) demonstrated that S-1
was non-inferior to GEM, but did not prove the superiority of
combination therapy with GEM and S-1 (3). Therefore, GEM or S-1 is recommended for
standard chemotherapy of advanced or recurrent pancreatic cancer
(APC).
The incidence of myelosuppression, such as
neutropenia, in first-line GEM therapy is high, which may delay
treatment and affect prognosis. Moreover, neutrophil count
(4,5)
and the ratio of neutrophils to lymphocytes (6,7) have been
reported to be prognostic factors for APC patients. In addition, it
has been reported that neutropenia is a prognostic factor in
gastric (8) and colon cancers
(9), as well as haematopoietic
tumours (10). However, due to the
poor prognosis of pancreatic cancer, the association between
neutropenia and prognosis, and details such as dose and relative
dose intensity (RDI), have not been investigated in the clinical
setting. Furthermore, in the GEST study (3), gastrointestinal symptoms such as nausea,
diarrhoea and stomatitis have been frequently observed among
adverse events (AEs) associated with S-1 monotherapy. Thus, when
administering S-1 as second-line therapy, tolerability to AEs may
be reduced, with deterioration of the patient's condition. The
frequency of AEs and treatment continuity associated with
second-line S-1 chemotherapy have not been extensively investigated
(11–14). We previously reported that albumin
(Alb) levels <3.5 g/dl and creatinine clearance levels <78
ml/min were risk factors for treatment discontinuation or dosage
reduction of S-1 in gastric cancer chemotherapy (15,16).
Therefore, this retrospective study aimed to
investigate the safety of S-1 as second-line therapy for APC
patients. In addition, we evaluated the association between
neutropenia occurring during first-line GEM therapy and
survival.
Subjects and methods
Subjects and methods
Between January, 2010 and December, 2014, 123
patients received chemotherapy for APC at the Ogaki Municipal
Hospital (Ogaki, Japan). Of those, 37 received GEM as first-line
and S-1 as second-line therapy (GEM→S-1 group). A further 60
patients received GEM as first-line therapy, but did not receive
second-line therapy (GEM group). Age, RDI, administration period,
AEs and reasons for dose reduction or temporary suspension of
medication were retrospectively surveyed for each patient. In
addition, patients receiving ongoing treatment with GEM or S-1
during the study period were excluded. The dates of AEs and reasons
for discontinuation of chemotherapy were extracted from electronic
charts. The severity of AEs was classified according to the Common
Terminology Criteria for Adverse Events, version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
The present study was approved by the Institutional Review Board of
Ogaki Municipal Hospital.
Doses and routes of GEM and S-1
therapies
GEM was administered intravenously at a starting
dose of 1,000 mg/m2 over 30 min, weekly, on days 1, 8
and 15 over a 4-week period. S-1 was orally administered for 4
weeks (dose: <1.25 m2 of body surface area, 80 mg/d;
1.25-1.5 m2, 100 mg/d; ≥1.5m2, 120 mg/d),
followed by a 2-week washout period.
Statistical analysis
The F-test was performed to compare the two groups.
Welch's t-test or the Chi-square test of independence (Fisher's
exact probability test) was used to analyse the patients'
characteristics (age, neutrophil count, RDI and dosage) shown in
Table I. The Kaplan-Meier log-rank
test was used to compare overall survival. In all these tests,
P<0.05 was considered to indicate statistically significant
differences. All statistical analyses were performed using JMP 8
software (SAS Institute Inc., Cary, NC, USA).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Patients who received
second-line treatment | Patients who did not
receive second-line treatment |
|
|---|
|
|
|
|
|
|---|
| Characteristics | GEM→S-1 (n=37) | GEM (n=60) | P-value |
|---|
| Age, years
(range) | 68 (54–77) | 66.3 (43–83) | 0.3738 |
| Gender, n |
|
| 0.4681 |
|
Female | 18 | 31 |
|
| Male | 19 | 29 |
|
| BSA,
m2 | 1.45 (1.12–1.93) | 1.48 (1.22–1.81) | 0.5942 |
| CrCl, ml/min | 74.5
(43.2–120.9) | 76.1
(21.7–150.4) | 0.9632 |
| Disease stage, n |
|
| 0.0845 |
| IVa | 19 | 21 |
|
| IVb | 18 | 39 |
|
| Disease status,
n |
|
| 0.0090 |
|
Unresectable | 22 | 50 |
|
|
Recurrent | 15 | 10 |
|
| Neutrophils, /µl | 3,650
(1,610–7,740) | 4,100
(1,800–9,290) | 0.0431 |
| RDI of GEM
(range) | 90.4 (36.9–100) | 83.4 (53.0–100) | 0.1162 |
| Administration period
of GEM, days (range) | 159 (48–574) | 95 (7–882) | 0.2759 |
| Dosage of GEM, % | 100 (74.6–100) | 100 (77.1–100) | 0.9264 |
| Metastatic site,
n |
|
| 0.2308 |
|
Liver | 12 | 25 |
|
| Lung | 4 | 3 |
|
|
Peritoneum | 2 | 10 |
|
| Lymph
nodes | 3 | 8 |
|
|
Other | 1 | 9 |
|
| Complications, n |
|
| 0.2959 |
|
Hypertension | 17 | 16 |
|
|
Hyperlipidaemia | 6 | 3 |
|
|
Diabetes | 13 | 16 |
|
|
Asthma | 5 | 1 |
|
Results
Patient characteristics
The patients' characteristics are shown in Table I. In the GEM→S-1 and GEM groups, the
median age was 68 years (range, 54–77 years) and 66.3 years (range,
43–83 years); the median neutrophil count was 3,650/µl (range,
1,610–7,740/µl) and 4,100/µl (range, 1,800–9,290/µl (P=0.0431); the
RDI was 90.4% (range, 36.9–100%) and 83.4% (range, 53.0–100%); and
the dosage used was 100% (range, 74.6–100%) and 100% (range,
77.1–100%), respectively.
Overall survival in the GEM→S-1 and
S-1 groups
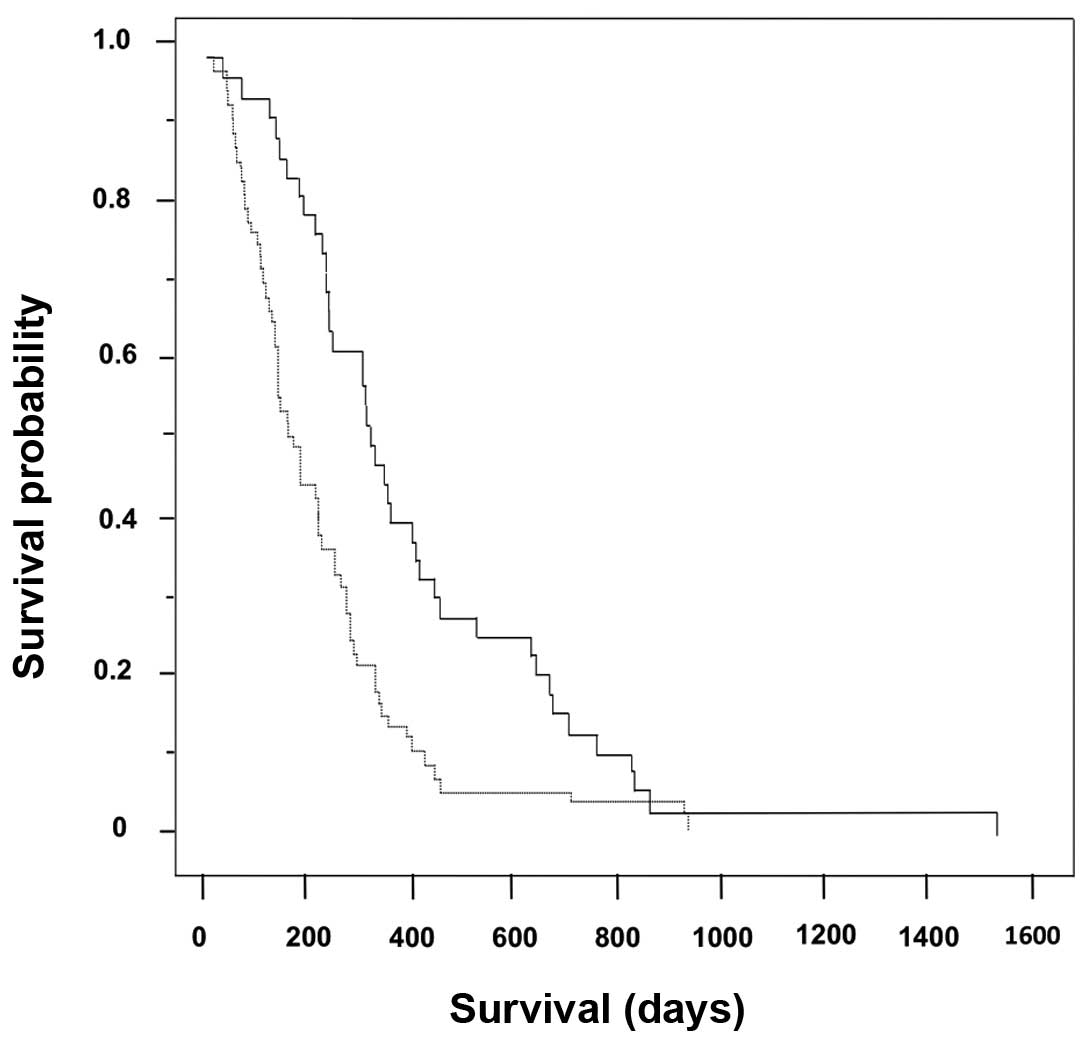
The Kaplan-Meier survival curves for the cohorts
(n=97) are shown in Fig. 1. The
median overall survival of the GEM→S-1 (n=37) and GEM (n=60) groups
were 323 days [95% confidence interval (CI): 138–218 days] and 172
days (95% CI: 105–184 days), respectively (log-rank test,
P=0.0004).
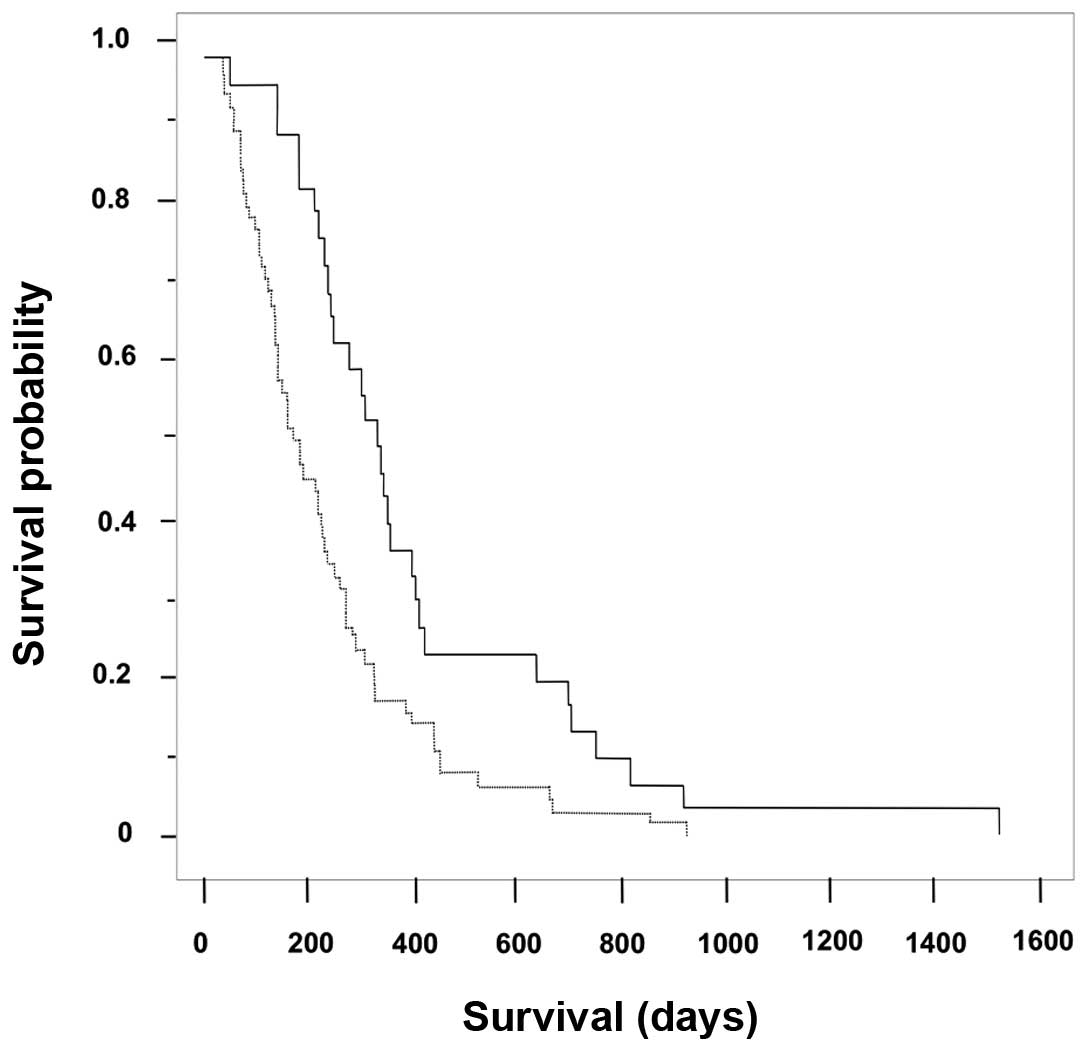
Overall survival according to the
highest grade of neutropenia following first-line therapy with
GEM
The Kaplan-Meier survival curves showing the highest
grade of neutropenia following first-line therapy with GEM (n=97)
are shown in Fig. 2. The median
overall survival time in the mild (grade ≤2; n=63) and severe
(grade ≥3; n=34) neutropenia groups was 178 days (95% CI: 182–275
days) and 330 days (95% CI: 297–514 days), respectively (log-rank
test, P=0.0023). In addition, the frequency of grade 3 or 4
neutropenia in the GEM→S-1 group (48.6%, 18/37 cases) was
significantly higher compared with that in the GEM group (26.7%,
16/60 cases; P=0.0238).
Reasons for discontinuation,
postponement and dose reduction
The reasons for discontinuation, postponement and
dose reduction in the GEM→S-1 and GEM groups are shown in Table II. In the GEM→S-1 group, GEM
administration was interrupted due to progressive disease (PD) in
36 cases, or AEs in 1 case. S-1 discontinuation occurred due to
changes in performance status (PS), PD, AEs (diarrhoea and
anorexia), and other reasons in 21, 13, 2 and 1 cases,
respectively. In addition, GEM administration was postponed due to
haematological and non-haematological toxicities in 22 and 4 cases,
respectively, and other reasons in 1 case. S-1 administration was
postponed due to haematological toxicities in 3 cases;
non-haematological toxicities in 6 cases (diarrhoea, stomatitis,
skin hyperpigmentation, constipation, anorexia and vomiting); a
decrease in PS in 2 cases; due to the patient's wishes in 1 case;
and other reasons in 3 cases.
 | Table II.Reasons for treatment discontinuation,
postponement and dose reduction. |
Table II.
Reasons for treatment discontinuation,
postponement and dose reduction.
| A, GEM→S-1 group |
|---|
|
|---|
| Reason for
discontinuation | Reason for
postponement | Reason for dose
reduction |
|---|
|
|
|
|---|
|
| GEM | S-1 |
| GEM | S-1 |
| GEM | S-1 |
|---|
| Progressive
disease | 36 | 13 | Adverse events |
|
| Decrease in PS | 2 | 7 |
|
|
|
|
Hematological toxicity | 22 | 3 | Myelosuppression | 8 | 1 |
| Adverse events | 1 | 2 |
Non-hematological
toxicity | 4 | 6 | Non-hematological
toxicity | 0 | 4 |
| Decrease in PS | 0 | 21 | Decrease in PS | 0 | 2 | Other | 2 | 0 |
| Other | 0 | 1 | Subject's wishes | 0 | 1 |
|
|
|
|
|
|
| Other | 1 | 3 |
|
|
|
|
| B, GEM group |
|
| Reason for
discontinuation | Reason for
postponement | Reason for dose
reduction |
|
|
|
| Progressive
disease |
| 36 | Adverse events |
|
| Decrease in PS |
| 8 |
|
|
|
|
Hematological toxicity |
| 27 |
Myelosuppression |
| 1 |
| Adverse events |
| 7 |
Non-hematological
toxicity |
| 21 | Non-hematological
toxicity |
| 0 |
| Decrease in PS |
| 14 | Decrease in PS |
| 4 | Renal function
degeneracy |
| 1 |
| Other |
| 6 | Other |
| 3 | Other |
| 0 |
In the GEM group, the dosage was reduced due to a
decrease in PS, myelosuppression, renal failure and other reasons
in 8, 1, 1 and 1 cases, respectively.
Main adverse events of second-line
therapy with S-1
The main AEs caused by second-line therapy with S-1
are shown in Table III. The most
common haematological toxicities were oligochromemia (14 cases,
37.8%), leukopenia (7 cases, 18.9%) and neutropenia (6 cases,
16.2%). Non-haematological toxicities included anorexia (7 cases,
18.9%), diarrhoea (7 cases, 18.9%), malaise (6 cases, 16.2%),
stomatitis (6 cases, 16.2%), nausea (5 cases, 13.5%), watery eyes
(5 cases, 13.5%) and skin hyperpigmentation (4 cases, 10.8%).
 | Table III.Adverse events following second-line
therapy with S-1. |
Table III.
Adverse events following second-line
therapy with S-1.
|
| Grade |
|
|
|---|
|
|
|
|
|
|---|
| Adverse events | 1 | 2 | 3 | 4 | All grades (%) | Grade ≥3 (%) |
|---|
| Oligochromemia | 1 | 9 | 4 | 0 | 14 (37.8) | 4 (10.8) |
| Leukopenia | 4 | 2 | 1 | 0 | 7 (18.9) | 1 (2.7) |
| Neutropenia | 2 | 2 | 2 | 0 | 6 (16.2) | 2 (5.4) |
| AST/ALT
increase | 3 | 0 | 1 | 0 | 4 (10.8) | 1 (2.7) |
| Blood bilirubin
increase | 2 | 1 | 0 | 0 | 3 (8.1) | 0 (0.0) |
| Creatinine
increase | 1 | 2 | 0 | 0 | 3 (8.1) | 0 (0.0) |
| Anorexia | 4 | 3 | 0 | 0 | 7 (18.9) | 0 (0.0) |
| Diarrhoea | 3 | 4 | 0 | 0 | 7 (18.9) | 0 (0.0) |
| Malaise | 4 | 2 | 0 | 0 | 6 (16.2) | 0 (0.0) |
| Stomatitis | 4 | 2 | 0 | – | 6 (16.2) | 0 (0.0) |
| Nausea | 3 | 1 | 1 | 0 | 5 (13.5) | 1 (2.7) |
| Watering eyes | 5 | 0 | 0 | – | 5 (13.5) | 0 (0.0) |
| Skin
hyperpigmentation | 4 | 0 | – | – | 4 (10.8) | 0 (0.0) |
| Rash | 3 | 0 | 0 | 0 | 3 (8.1) | 0 (0.0) |
| Hand-foot
syndrome | 2 | 0 | 1 | 0 | 3 (8.1) | 1 (2.7) |
| Oedema | 1 | 2 | 0 | 0 | 3 (8.1) | 0 (0.0) |
Association between the incidence of
gastrointestinal toxicity and serum Alb in second-line therapy with
S-1
Following second-line S-1 therapy in the GEM→S-1
group, the frequency of grade 2, 3 or 4 malaise and digestive
system disorders in subjects with Alb <3.5 g/dl (10/14 cases)
were significantly higher compared with those with Alb ≥3.5 g/dl
(2/23 cases; P=0.0002). In patients where treatment was interrupted
due to diarrhoea and nausea (2 cases), the Alb levels were 3.3 and
3.2 g/dl, respectively. In addition, S-1 therapy was postponed in 6
cases due to AEs such as diarrhoea, stomatitis, skin
hyperpigmentation, anorexia and nausea. In 5 of 6 of these cases,
the Alb level was ≤3.5 g/dl.
Discussion
The aim of this retrospective study was to
investigate the safety of S-1 as second-line therapy, and to
evaluate the association between neutropenia occurring during
first-line GEM therapy and survival in APC patients.
It has been reported that second-line treatment with
S-1 monotherapy is associated with a better prognosis in APC
patients (4,12–14).
Similarly, this study has demonstrated that it is important to use
GEM and S-1 for the treatment of APC. Furthermore, a study by
Shitara et al (8) reported
that neutropenia occurring during weekly paclitaxel treatment
administered as second-line therapy to advanced gastric cancer
patients is strongly associated with a better prognosis. In this
study, the prognosis of APC patients with grade ≥3 neutropenia
during first-line GEM therapy was good.
Regarding the association between neutropenia and
prognosis, Shitara et al (8)
hypothesized that neutropenia, an indicator of bone marrow
suppression caused by a specific dose of a chemotherapeutic agent,
may also be a surrogate marker indicating that the same dose is
adequate for exerting an antitumor effect. If neutropenia is not
present, it is possible that the patient has been administered too
low a dose. In our study, no significant differences were found
between the GEM→S-1 and GEM groups with regard to RDI and dose, or
when the dosages were reduced. However, the neutrophil count was
high in the GEM group at the start of treatment. Fridlender et
al (17) reported that
neutrophils are involved in vascularisation and are associated with
cancer metastasis and angiogenesis. Hatori et al (4) reported that the number of neutrophils
present prior to the first GEM treatment is a prognostic factor.
Additionally, it has been reported that pharmacodynamics rather
than pharmacokinetics determines the effect of GEM on survival.
Therefore, we recommend avoiding dosage reduction
when the neutrophil count is high. These findings may aid future
evaluation of dose escalation in patients without neutropenia to
prolong survival. Prospective trials are required to assess whether
dosing adjustments based on neutropenia may improve
chemotherapeutic efficacy.
Regarding the safety of second-line therapy with
S-1, grade ≥3 haematological toxicities were observed, but
non-haematological toxicities were rarely recorded. The main AEs
observed following second-line therapy with S-1 included
haematological toxicities, such as oligochromemia (37.8%),
leukopenia (18.9%) and neutropenia (16.2%), and non-haematological
toxicities, such as anorexia (18.9%), diarrhoea (18.9%), malaise
(16.2%) and stomatitis (16.2%), which were also reported by Todaka
et al (18). In addition, of
the 37 patients who received S-1 therapy, treatment was
discontinued in 2 cases due to non-haematological toxicities
(diarrhoea and anorexia). By contrast, in 6 cases with
non-haematological toxicities, such as diarrhoea, stomatitis,
hand-foot syndrome, constipation, anorexia and vomiting, S-1
therapy was safely continued by postponing treatment. However,
during second-line therapy with S-1, the frequency of grade ≥2
fatigue and gastrointestinal toxicity was 27.0% (10/37 cases) in
patients with Alb levels <3.5 g/dl. This result is similar to
that of previous studies (15,16) and
should be considered when treating patients with S-1, as it may
affect the treatment course. Similarly, fatigue and
gastrointestinal toxicity in APC chemotherapy patients with Alb
levels <3.5 g/dl must be carefully considered when planning the
chemotherapy protocol.
In conclusion, neutropenia occurring when GEM is
administered as first-line treatment to APC patients is strongly
associated with a better prognosis. S-1 therapy as second-line
treatment has been associated with a low incidence of severe AEs
and the patients were able to successfully continue treatment.
References
|
1
|
Matsuda T, Ajiki W, Marugame T, Ioka A,
Tsukuma H and Sobue T: Research Group of Population-Based Cancer
Registries of Japan: Population-based survival of cancer patients
diagnosed between 1993 and 1999 in Japan: A chronological and
international comparative study. Jpn J Clin Oncol. 41:40–51. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997.PubMed/NCBI
|
|
3
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hatori M, Tsuji D, Taku K, Daimon T,
Kamezato M, Ikeda M, Makuta R, Hayashi H, Inoue K and Itoh K:
Prognostic factors in patients with unresectable pancreatic cancer
treated with gemcitabine: a retrospective analysis. Jpn J Pharm
Health Care Sci. 40:734–741. 2014. View Article : Google Scholar
|
|
5
|
Teramukai S, Kitano T, Kishida Y, Kawahara
M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al:
Pretreatment neutrophil count as an independent prognostic factor
in advanced non-small-cell lung cancer: An analysis of Japan
Multinational Trial Organisation LC00-03. Eur J Cancer.
45:1950–1958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
An X, Ding PR, Li YH, Wang FH, Shi YX,
Wang ZQ, He YJ, Xu RH and Jiang WQ: Elevated neutrophil to
lymphocyte ratio predicts survival in advanced pancreatic cancer.
Biomarkers. 15:516–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stotz M, Gerger A, Eisner F, Szkandera J,
Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewiesn FS, Lackner
C, et al: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with primary operable and inoperable
pancreatic cancer. Br J Cancer. 109:416–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shitara K, Matsuo K, Takahari D, Yokota T,
Shibata T, Ura T, Ito S, Sawaki A, Tajika M, Kawai H and Muro K:
Nuetropenia as a prognostic factor in advanced gastric cancer
patients undergoing second-line chemotherapy with weekly
paclitaxel. Ann Oncol. 21:2403–2409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shitara K, Matsuo K, Oze I, Mizota A,
Kondo C, Nomura M, Yokota T, Takahari D, Ura T and Muro K:
Meta-analysis of neutropenia or leukopenia as a prognostic factor
in patients with malignant disease undergoing chemotherapy. Cancer
Chemother Pharmacol. 68:301–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shitara K, Matsuo K, Takahari D, Yokota T,
Inaba Y, Yamaura H, Sato Y, Najima M, Ura T and Muro K:
Neutropaenia as prognostic factor in metastatic colorectal cancer
patients undergoing chemotherapy with first-line FOLFOX. Eur J
Cancer. 45:1757–1763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawashima H, Itoh A, Ohno E, Nakamura M,
Miyahara R, Ohmiya N, Hara K, Kanamori A, Itoh T, Taki T, et al:
Prospective multicenter study to investigate the introduction rate
of second-line S-1 in gemcitabine-refractory unresectable
pancreatic cancer. Cancer Chemother Pharmacol. 68:677–683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakai Y1, Isayama H, Sasaki T, Sasahira N,
Kogure H, Hirano K, Tsujino T, Ijichi H, Tateishi K, Tada M, et al:
Impact of S-1 in patients with gemcitabine-refractory pancreatic
cancer in Japan. Jpn J Clin Oncol. 40:774–780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakai Y1, Isayama H, Sasaki T, Sasahira N,
Ito Y, Kogure H, Togawa O, Matsubara S, Arizumi T, Yagioka H, et
al: Impact of S-1 on the survival of patients with advanced
pancreatic cancer. Pancreas. 39:989–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takasawa O, Fujita N, Noda Y, Kobayashi G,
Ito K, Obana T, Horaguchi J, Koshita S, Kanno Y, Suzuki T, et al:
Second-line treatment with S-1 in advanced pancreatic cancer.
Gastroenterology. 48:207–213. 2009.
|
|
15
|
Kimura M, Morihata K, Ito D, Iwai M, Okada
K, Usami E, Nakao T, Yoshimura T and Yasuda T: Continuous
administration and safety of S-1 in adjuvant chemotherapy for
gastric cancer. Cancer & chemotherapy. 37:829–834. 2010.(In
Japanese).
|
|
16
|
Kimura M, Usami E, Yoshimura T, Yasuda T,
Kaneoka Y, Teramachi H, Sugiyama T and Tsuchiya T: Pharmaceutical
care for patients undergoing S-1 plus cisplatin therapy for
unresectable recurrent gastric cancer. J Pharm Pract. 26:409–414.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fridlender ZG and Albelda SM:
Tumor-associated neutrophils: Friend or foe? Carcinogenesis.
33:949–955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Todaka A, Fukutomi A, Boku N, Onozawa Y,
Hironaka S, Yasui H, Yamazaki K, Taku K, Machida N, Sakamoto T and
Tomita H: S-1 monotherapy as second-line treatment for advanced
pancreatic cancer after gemcitabine failure. Jpn J Clin Oncol.
40:567–572. 2010. View Article : Google Scholar : PubMed/NCBI
|