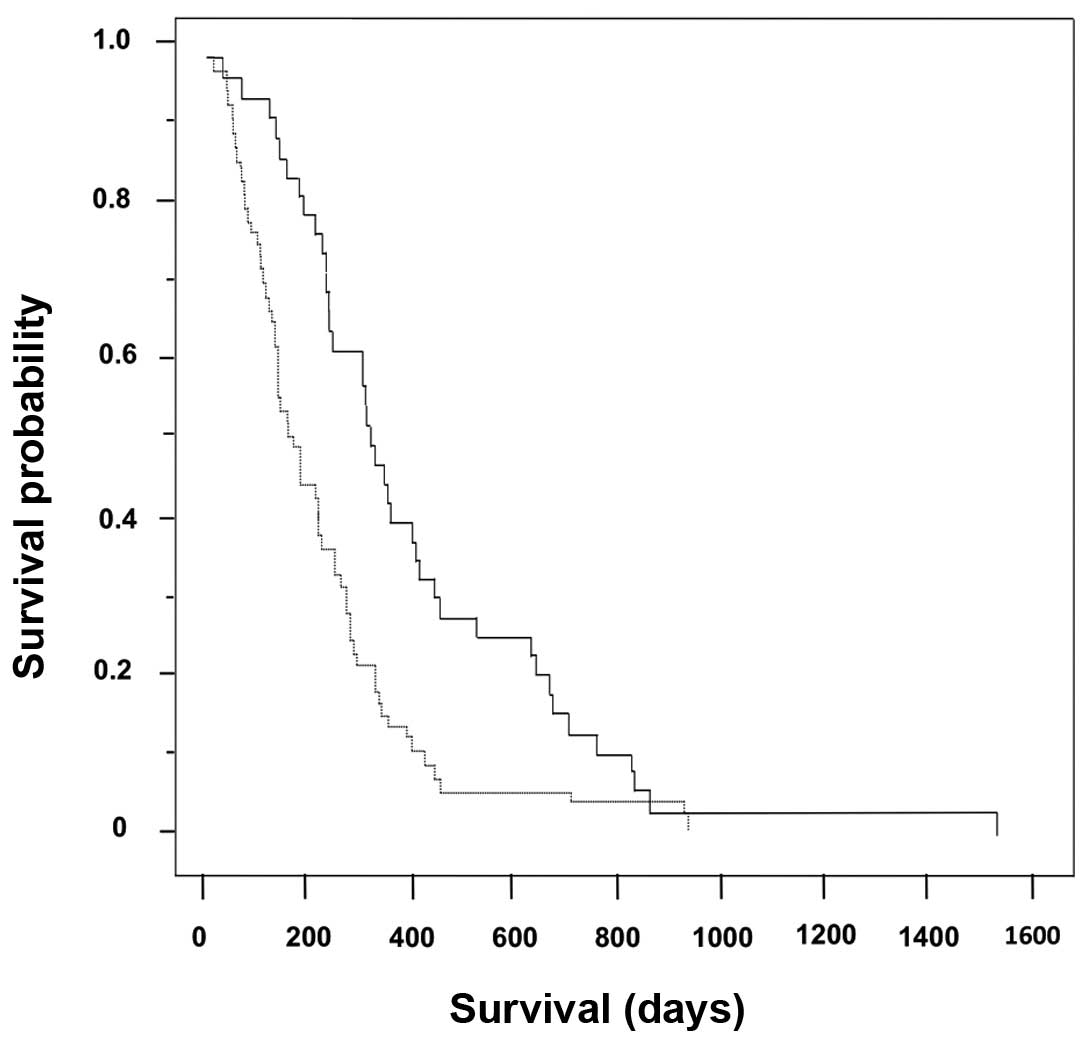
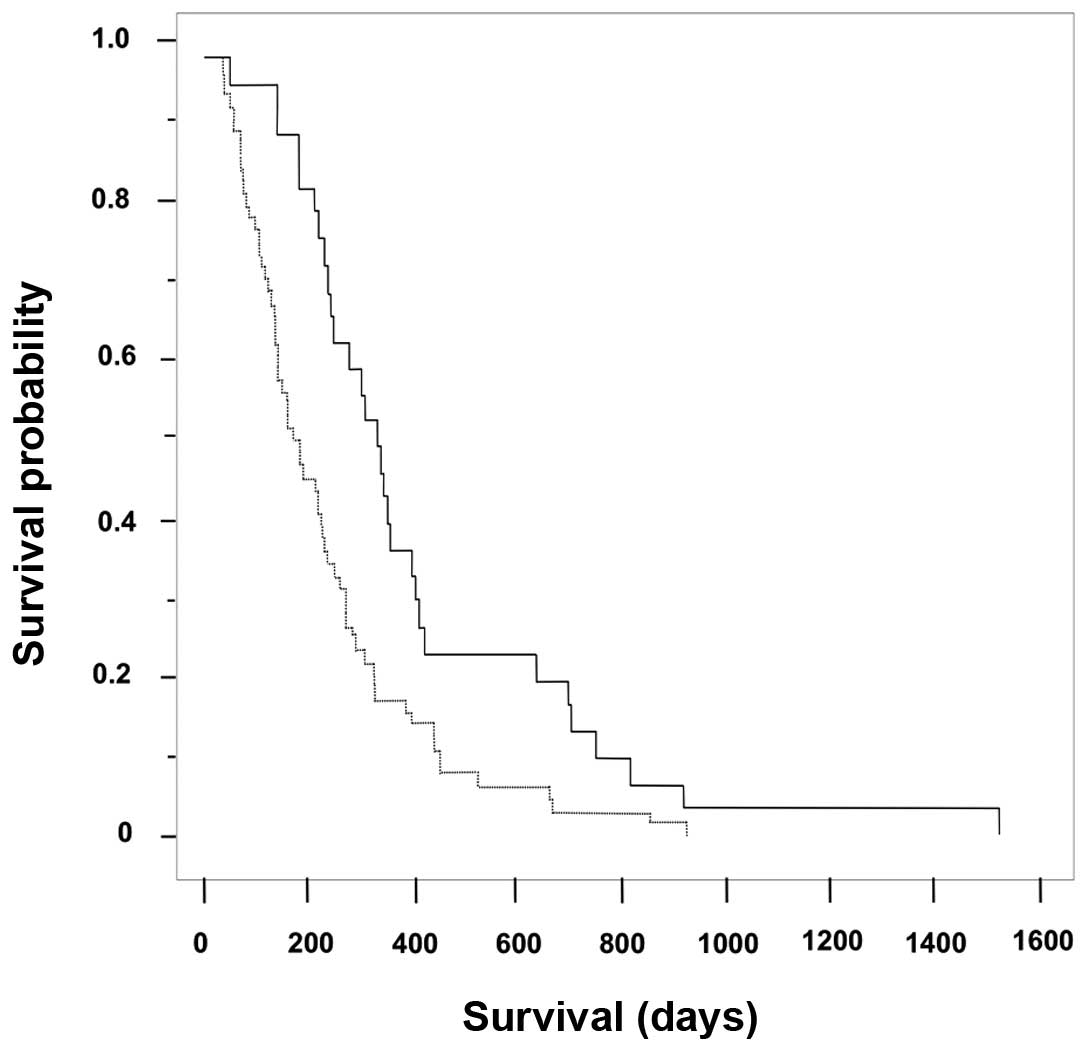
|
1
|
Matsuda T, Ajiki W, Marugame T, Ioka A,
Tsukuma H and Sobue T: Research Group of Population-Based Cancer
Registries of Japan: Population-based survival of cancer patients
diagnosed between 1993 and 1999 in Japan: A chronological and
international comparative study. Jpn J Clin Oncol. 41:40–51. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997.PubMed/NCBI
|
|
3
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hatori M, Tsuji D, Taku K, Daimon T,
Kamezato M, Ikeda M, Makuta R, Hayashi H, Inoue K and Itoh K:
Prognostic factors in patients with unresectable pancreatic cancer
treated with gemcitabine: a retrospective analysis. Jpn J Pharm
Health Care Sci. 40:734–741. 2014. View Article : Google Scholar
|
|
5
|
Teramukai S, Kitano T, Kishida Y, Kawahara
M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al:
Pretreatment neutrophil count as an independent prognostic factor
in advanced non-small-cell lung cancer: An analysis of Japan
Multinational Trial Organisation LC00-03. Eur J Cancer.
45:1950–1958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
An X, Ding PR, Li YH, Wang FH, Shi YX,
Wang ZQ, He YJ, Xu RH and Jiang WQ: Elevated neutrophil to
lymphocyte ratio predicts survival in advanced pancreatic cancer.
Biomarkers. 15:516–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stotz M, Gerger A, Eisner F, Szkandera J,
Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewiesn FS, Lackner
C, et al: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with primary operable and inoperable
pancreatic cancer. Br J Cancer. 109:416–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shitara K, Matsuo K, Takahari D, Yokota T,
Shibata T, Ura T, Ito S, Sawaki A, Tajika M, Kawai H and Muro K:
Nuetropenia as a prognostic factor in advanced gastric cancer
patients undergoing second-line chemotherapy with weekly
paclitaxel. Ann Oncol. 21:2403–2409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shitara K, Matsuo K, Oze I, Mizota A,
Kondo C, Nomura M, Yokota T, Takahari D, Ura T and Muro K:
Meta-analysis of neutropenia or leukopenia as a prognostic factor
in patients with malignant disease undergoing chemotherapy. Cancer
Chemother Pharmacol. 68:301–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shitara K, Matsuo K, Takahari D, Yokota T,
Inaba Y, Yamaura H, Sato Y, Najima M, Ura T and Muro K:
Neutropaenia as prognostic factor in metastatic colorectal cancer
patients undergoing chemotherapy with first-line FOLFOX. Eur J
Cancer. 45:1757–1763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawashima H, Itoh A, Ohno E, Nakamura M,
Miyahara R, Ohmiya N, Hara K, Kanamori A, Itoh T, Taki T, et al:
Prospective multicenter study to investigate the introduction rate
of second-line S-1 in gemcitabine-refractory unresectable
pancreatic cancer. Cancer Chemother Pharmacol. 68:677–683. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakai Y1, Isayama H, Sasaki T, Sasahira N,
Kogure H, Hirano K, Tsujino T, Ijichi H, Tateishi K, Tada M, et al:
Impact of S-1 in patients with gemcitabine-refractory pancreatic
cancer in Japan. Jpn J Clin Oncol. 40:774–780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakai Y1, Isayama H, Sasaki T, Sasahira N,
Ito Y, Kogure H, Togawa O, Matsubara S, Arizumi T, Yagioka H, et
al: Impact of S-1 on the survival of patients with advanced
pancreatic cancer. Pancreas. 39:989–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takasawa O, Fujita N, Noda Y, Kobayashi G,
Ito K, Obana T, Horaguchi J, Koshita S, Kanno Y, Suzuki T, et al:
Second-line treatment with S-1 in advanced pancreatic cancer.
Gastroenterology. 48:207–213. 2009.
|
|
15
|
Kimura M, Morihata K, Ito D, Iwai M, Okada
K, Usami E, Nakao T, Yoshimura T and Yasuda T: Continuous
administration and safety of S-1 in adjuvant chemotherapy for
gastric cancer. Cancer & chemotherapy. 37:829–834. 2010.(In
Japanese).
|
|
16
|
Kimura M, Usami E, Yoshimura T, Yasuda T,
Kaneoka Y, Teramachi H, Sugiyama T and Tsuchiya T: Pharmaceutical
care for patients undergoing S-1 plus cisplatin therapy for
unresectable recurrent gastric cancer. J Pharm Pract. 26:409–414.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fridlender ZG and Albelda SM:
Tumor-associated neutrophils: Friend or foe? Carcinogenesis.
33:949–955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Todaka A, Fukutomi A, Boku N, Onozawa Y,
Hironaka S, Yasui H, Yamazaki K, Taku K, Machida N, Sakamoto T and
Tomita H: S-1 monotherapy as second-line treatment for advanced
pancreatic cancer after gemcitabine failure. Jpn J Clin Oncol.
40:567–572. 2010. View Article : Google Scholar : PubMed/NCBI
|