Introduction
Chondroma is a benign tumor, originating in
chondrocytes. It is common in long bone, but rarely occurs in the
lung parenchyma. The clinicopathological features of pulmonary
chondroma has been rarely reported (1,2). Bateson
(3) distinguished endobronchial
chondroma from pulmonary chondroma based on the histogenesis of
lung chondroma. The former grows into the bronchial lumen and
accounts for <25% of all benign cartilage neoplasms of the lung.
The latter grows into lung parenchyma and has an incidence of about
0.1% of all benign lung tumor types. Between July 2003 and June
2015, 29 patients with pulmonary chondroma underwent surgical
operation at The Affiliated Hospital of North Sichuan Medical
College (Nanchong, China). All patients were pathologically
diagnosed. The present paper aimed to summarize the
clinicopathological features and the outcome of surgical treatment
of pulmonary chondroma. Additionally, the present study clarified
the differences between pulmonary chondroma and other benign
pulmonary tumor.
Materials and methods
Clinical data
The present study was approved by the Ethics
Committee of North Sichuan Medical College (Nanchong, China). All
patients provided written informed consent. The clinical data for
each of the 29 patients is shown in Table
I. A total of 29 patients were pathologically diagnosed
pulmonary chondroma, including 16 males and 13 females, with an
average age of 57 years (range, 39–78-years-old). Of these
patients, 18 exhibited no clinical symptoms and the pulmonary
chondroma was detected by routine medical examination, 7 patients
were characterized by coughing, hemoptysis, shortness of breath and
other symptoms, only 3 patients presented with chest pain as the
predominant symptom and 1 patient with esophageal cancer was
identified by preoperative examination. Physical examination
revealed only 3 patients with low breath sounds and others without
obvious abnormalities. Lung tumor-like lesions, nodules and varying
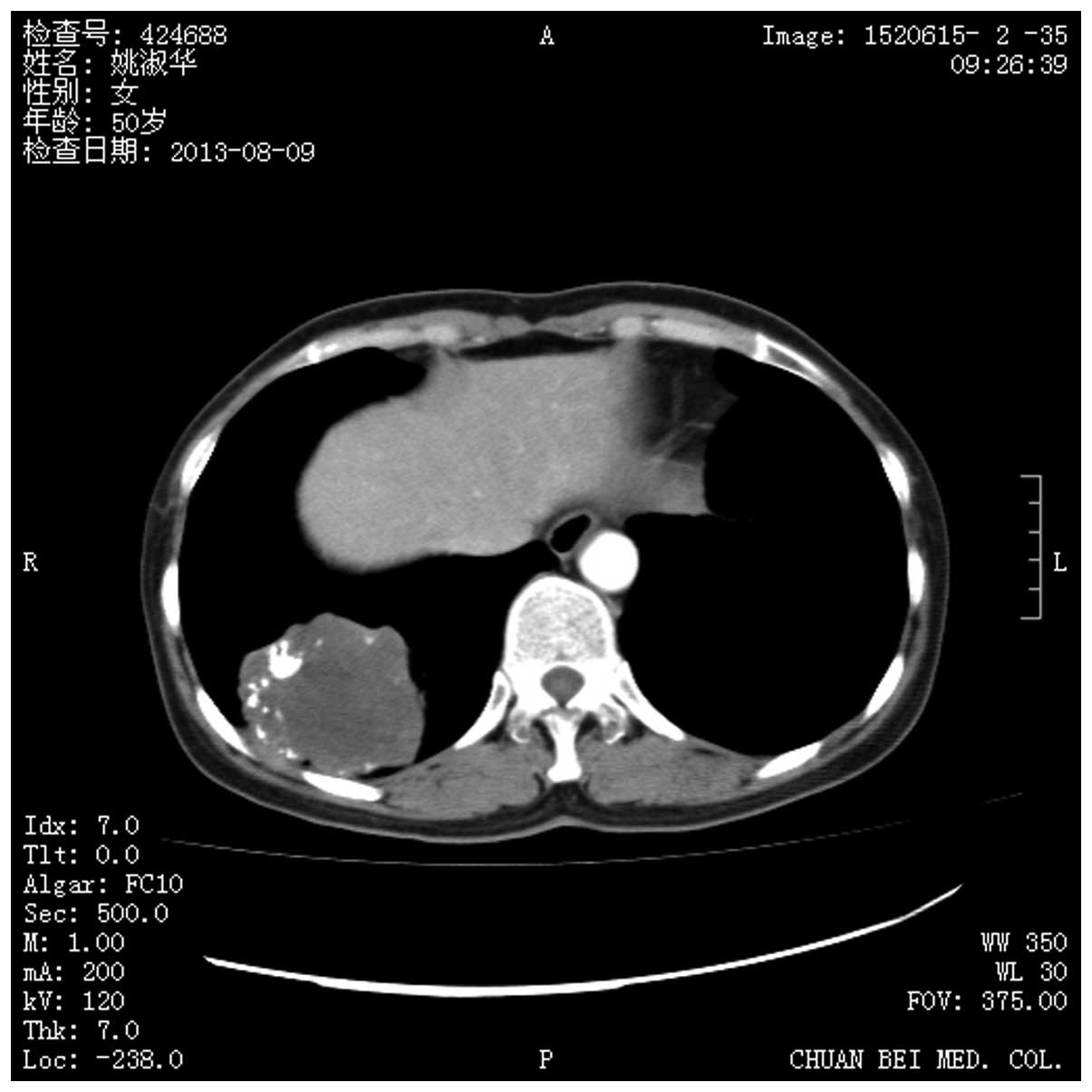
degrees of calcification was demonstrated by chest computed
tomography (CT) scan (Fig. 1). No
significant enlargement of the lymph nodes and pulmonary cavity was
observed. Pulmonary masses of 9 patients were in the right lower
lobe, 2 were in the right upper lobe, 3 were in the right middle
lobe, 11 were in the left upper lobe and 4 were in the left lower
lobe. The mean tumor diameter was 3.6 cm, ranging between 1.0 cm
and 8.5 cm. The edge of the 8 cases were rough and the rest were
smooth. No obvious abnormalities were revealed by other routine
preoperative examinations, including head CT scan, radionuclide
bone scan and abdominal ultrasound. Preoperatively, 13 patients
were considered hamartoma, 14 patients were considered benign
nodules, only 2 patients with chest wall adhesion were suspected of
malignant infiltration and no patient was diagnosed pulmonary
chondroma.
 | Table I.Clinical data. |
Table I.
Clinical data.
| Patient no. | Age (years) | Gender | Size (cm) | Operative time
(min) | Blood loss (ml) | Drainage (days) | Follow-up | Position | Preoperative
diagnosis |
|---|
| 1 | 51 | M | 2.3 | 64 | 45 | 3 | Alive without
recurrence | Right lower lobe | Hamartoma |
| 2 | 43 | F | 4.6 | 78 | 30 | 4 | Alive without
recurrence | Right lower lobe | Benign nodules |
| 3 | 38 | M | 5.2 | 155 | 10 | 2 | Succumbed to
non-neoplastic diseases | Right upper lobe | Hamartoma |
| 4 | 62 | M | 1.4 | 92 | 100 | 2 | Alive without
recurrence | Right middle
lobe | Benign nodules |
| 5 | 53 | M | 1 | 146 | 60 | 3 | Alive without
recurrence | Right lower lobe | Hamartoma |
| 6 | 68 | F | 3.9 | 104 |
5 | 3 | Alive without
recurrence | Left lower lobe | Hamartoma |
| 7 | 56 | F | 4.4 | 132 | 50 | 3 | Alive without
recurrence | Right upper lobe | Benign nodules |
| 8 | 57 | F | 2.3 | 141 | 120 | 2 | Succumbed to
non-neoplastic diseases | Right lower lobe | Hamartoma |
| 9 | 72 | F | 6.8 | 137 | 80 | 2 | Alive without
recurrence | Left upper lobe | Benign nodules |
| 10 | 52 | M | 4.1 | 48 | 30 | 2 | Alive without
recurrence | Right lower lobe | Hamartoma |
| 11 | 47 | F | 2.6 | 164 | 90 | 4 | Lost | Right lower lobe | Malignant tumor |
| 12 | 78 | M | 6.9 | 143 | 180 | 3 | Alive without
recurrence | Left upper lobe | Benign nodules |
| 13 | 64 | M | 1.8 | 156 | 40 | 4 | Alive without
recurrence | Left upper lobe | Benign nodules |
| 14 | 73 | F | 3 | 123 | 30 | 2 | Lost | Right middle
lobe | Hamartoma |
| 15 | 66 | M | 2.5 | 157 | 75 | 5 | Alive without
recurrence | Left lower lobe | Benign nodules |
| 16 | 69 | F | 5 | 129 | 350 | 2 | Alive without
recurrence | Right lower lobe | Benign nodules |
| 17 | 64 | M | 8.5 | 92 | 40 | 3 | Succumbed to
non-neoplastic diseases | Right middle
lobe | Benign nodules |
| 18 | 57 | F | 3.8 | 201 | 60 | 2 | Alive without
recurrence | Left upper lobe | Benign nodules |
| 19 | 52 | F | 3.2 | 107 | 80 | 8 | Alive without
recurrence | Left lower lobe | Hamartoma |
| 20 | 55 | M | 1.9 | 215 | 240 | 3 | Alive without
recurrence | Right lower lobe | Hamartoma |
| 21 | 58 | M | 1.8 | 104 | 30 | 4 | Alive without
recurrence | Left upper lobe | Malignant tumor |
| 22 | 46 | F | 3.7 | 106 | 50 | 3 | Succumbed to
non-neoplastic diseases | Left upper
lobe | Hamartoma |
| 23 | 70 | F | 2.2 | 192 | 40 | 3 | Alive without
recurrence | Left lower
lobe | Benign nodules |
| 24 | 51 | M | 3.2 | 104 | 45 | 3 | Alive without
recurrence | Left upper
lobe | Benign nodules |
| 25 | 48 | M | 1.2 | 174 | 100 | 2 | Alive without
recurrence | Left upper
lobe | Hamartoma |
| 26 | 59 | F | 7.4 | 117 | 80 | 5 | Alive without
recurrence | Right lower
lobe | Hamartoma |
| 27 | 54 | M | 2.3 | 94 | 100 | 3 | Alive without
recurrence | Left upper
lobe | Benign nodules |
| 28 | 43 | M | 4.2 | 89 | 160 | 2 | Alive without
recurrence | Left upper
lobe | Benign nodules |
| 29 | 47 | M | 3.2 | 90 | 55 | 3 | Alive without
recurrence | Left upper
lobe | Hamartoma |
Operative technique
The thoracotomy pneumonectomy was performed under
general anesthesia with single-lung ventilation, which may be
accomplished with double-lumen endotracheal tubes. The patients
were placed in the lateral decubitus position with the upper arm
suspended on a crossbar. Firstly, a 1.5 cm incision was placed in
the seventh intercostal space at mid axillary line. The pleural
cavity was entered to explore the lesion and surrounding
structures. Another incision (~7–9 cm long) was made in the fourth
intercostal space under the armpit if there was no adhesion, or
else anterolateral incision was used. During conventional
thoracotomy, the mass revealed no malignant cells by intraoperative
frozen section examination and was therefore confirmed as benign
tumors.
Statistical analysis
The data were analyzed using SPSS 22.0 (IBM SPSS,
Chicago, IL, USA) and the results were presented as the mean ±
standard deviation.
Results
A total of 29 patients were pathologically diagnosed
with pulmonary chondroma, including 16 males and 13 females, with
an average age of 57 years (range, 39–78 years). Of these patients,
18 exhibited no clinical symptoms and the pulmonary chondroma was
detected by routine medical examination, 7 were characterized by
coughing, hemoptysis, shortness of breath and other symptoms, 3
patients presented with chest pain as the predominant symptom and 1
patient with esophageal cancer was identified by preoperative
examination. Physical examination revealed only 3 patients with low
breath sounds and others without obvious abnormalities. Lung
tumor-like lesions, nodules and varying degrees of calcification
was demonstrated by chest computed tomography (CT) scan (Fig. 1). No significant enlargement of the
lymph nodes and pulmonary cavity was observed. Pulmonary masses of
9 patients were found in the right lower lobe, 2 were in the right
upper lobe, 3 were in the right middle lobe, 11 were in the left
upper lobe and 4 were in the left lower lobe. The mean tumor
diameter was 3.6 cm, ranging between 1 and 8.5 cm. The edge of the
8 cases were rough and the rest were smooth. No obvious
abnormalities were revealed by other routine preoperative
examinations, including head CT scan, radionuclide bone scan and
abdominal ultrasound. Preoperatively, 13 patients were considered
hamartoma, 14 patients were considered benign nodules, 2 patients
with chest wall adhesion were suspected of malignant infiltration
and no patient was diagnosed with pulmonary chondroma.
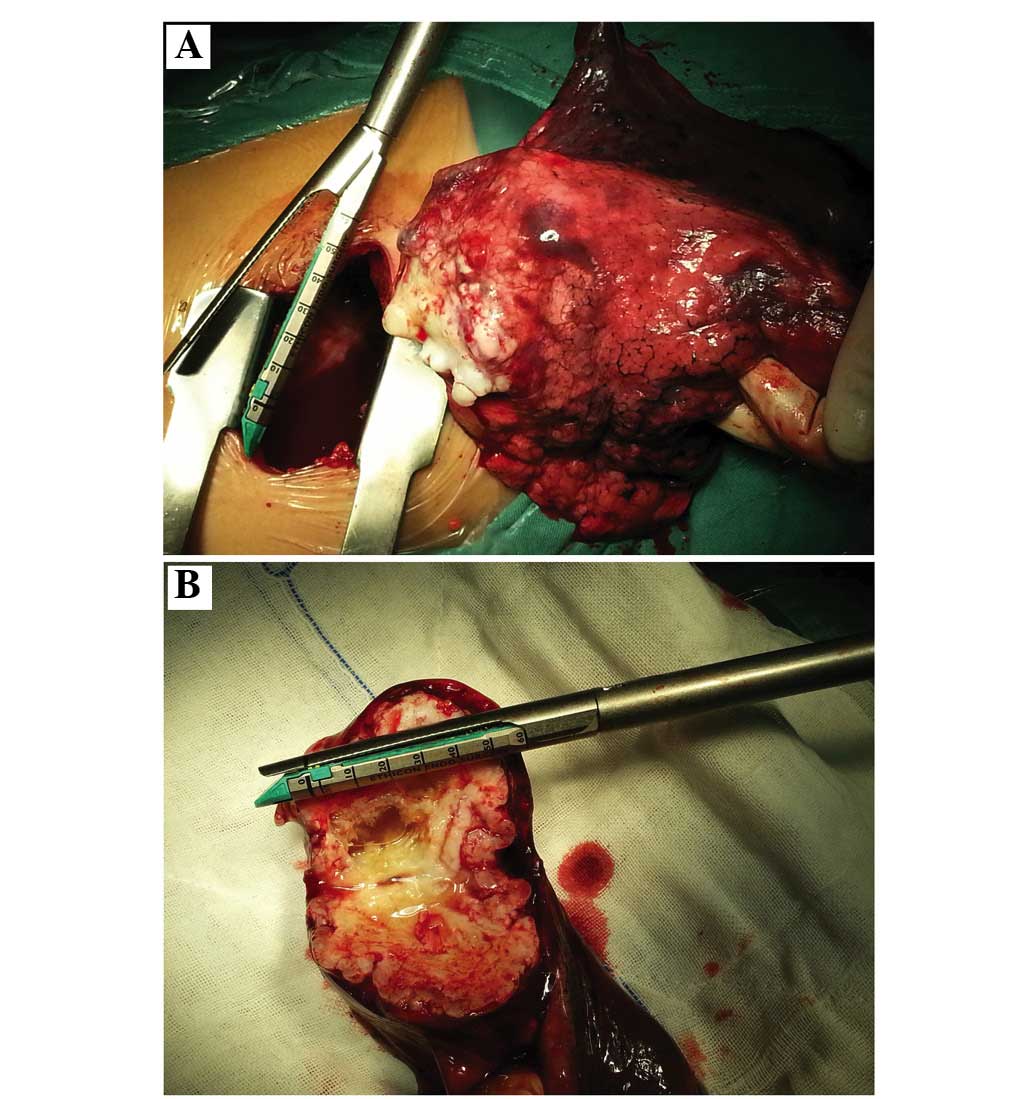
A total of 11 patients underwent lobectomy (Fig. 2), 17 patients underwent segmentectomy
and 1 patient used lump stripping. They were postoperatively
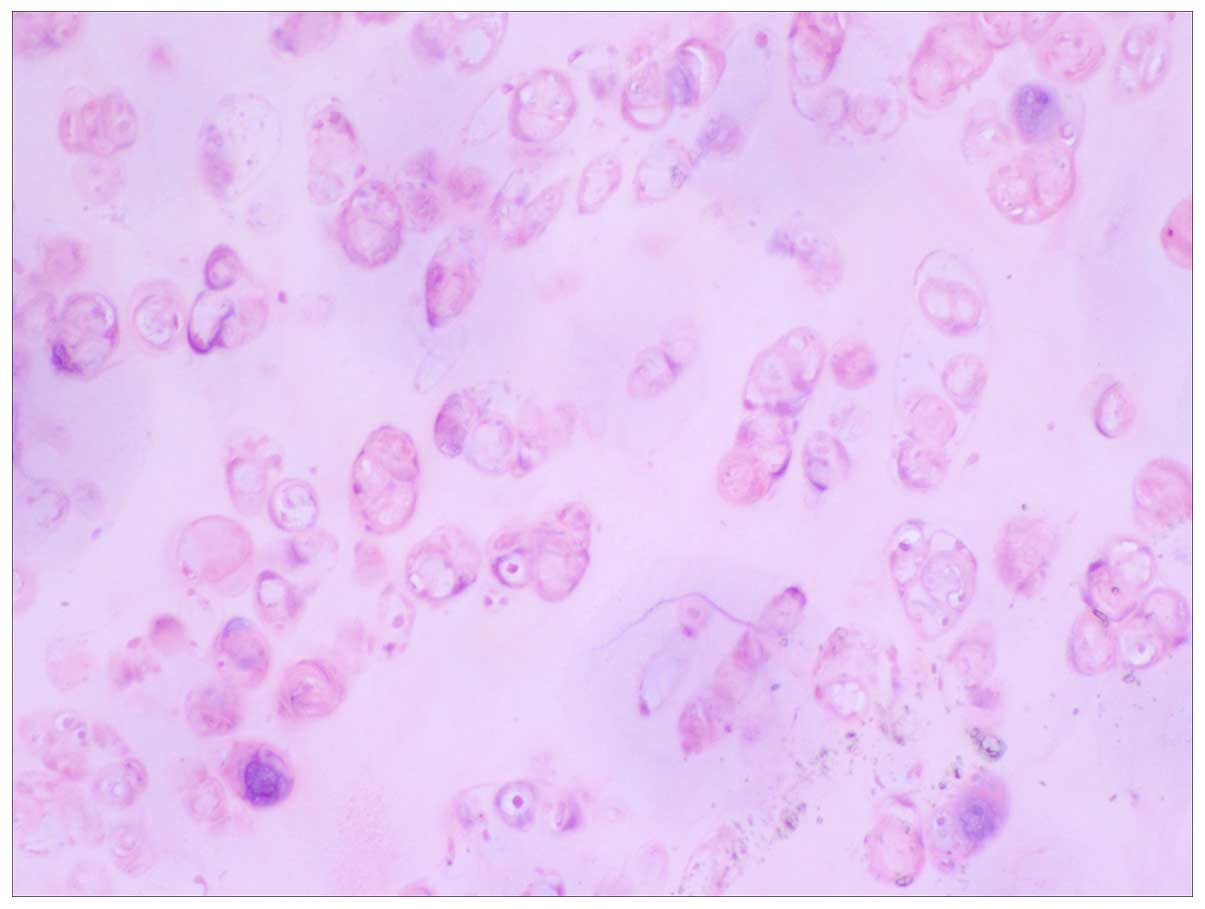
pathologically diagnosed as pulmonary chondroma (Fig. 3). All the lymph nodes were reactive
hyperplasia. Carney's triad was excluded by abdominal magnetic
resonance imaging (MRI) and gastroscopy. No mortality occurred
during surgery. The operative duration ranged between 48 and 215
min (mean, 126±22 min). The estimated blood loss ranged between 5
and 350 ml (mean, 82±23 ml). Additionally, no patient required a
blood transfusion. All patients, with the exception of 5 patients,
had an uneventful postoperative course (82.8%). Of the five
complications, two were postoperative encapsulated pleural effusion
and three were pulmonary infection. These 5 patients recovered well
following percutaneous catheter drainage by CT-guided and
anti-infection therapy. The drainage duration ranged between 2 and
8 days (mean, 3.1±1.8 days) and the postoperative hospital duration
ranged between 4 and 13 days (mean, 4.0±2.1 days). Patients were
followed-up between 2 and 135 months. During follow-up, 23 patients
were alive without recurrence, 1 patient succumbed to esophageal
cancer after 19 months post-surgery, 3 patients succumbed to other
diseases. A total of 2 patients were lost during follow-up.
Discussion
Pulmonary chondroma is often originated from ectopic
cartilage of lung tissue during embryonic development. Chondrocytes
in other parts of the tissues flowed into the lungs by bloodstream.
Connective tissues, reticulocytes developed into original direction
by certain stimulate conditions, became the embryo of mesenchymal
tissue, and then developed into chondrocytes. These are theoretical
speculations (2).
The tumor tissues were pale and translucent, hard
and lobulated on the lateral section. Under the microscope, the
tumors were observed to be formed by differentiation of mature
cartilage tissue, wrapped around the cartilage matrix. Cartilage
tissues can be hyaline cartilage, elastic cartilage and fibrous
cartilage, or diverse cartilage mixed together without other
mesenchymal tissue components, abnormal mitotic and adipose
tissues. Chondrocytes can encounter calcification, ossification and
mucoid degeneration (4).
A few case reports have identified pulmonary
chondroma (1,2). Only 0.04% of lung neoplasms were
identified to be pulmonary chondroma (3). Pulmonary chondroma was common in adult
females of 40–50-years-old and neonatal cases were occasionally
reported (2). However, in the present
group, 16 males and 13 females with an average age of 57 years
(range, 39–78-years-old), different from the literature (2,4,5). Pulmonary chondroma may occur in each
pulmonary lobe, particularly the right lower lobe. Of the 29
patients, 9 exhibited pulmonary chondroma located in the right
lower lobe and 15 were located in left lung, also inconsistent with
previous literature. The mean diameter of pulmonary chondroma was
2.8 cm and was usually asymptomatic (4). In the present study, the average
diameter was 3.6 cm (1.0–8.5 cm). Of the patients, 18 presented
without any clinical symptoms, 1 with esophageal cancer was
identified by preoperative examination. The symptoms of pulmonary
chondroma depended on the size and location of the tumor. If the
bronchus were oppressed, atelectasis was caused. Respiratory
symptoms, including cough with sputum or hemoptysis and shortness
of breath, can occur (6,7). In the present study, 7 cases (13.8%)
occurred. In addition, 3 patients exhibited chest pain. From the CT
scan, the tumor size in all three patients were larger and
exhibited chest wall invasion. Intraoperatively, the tumor shrunk
without invasion into the chest wall. As a result of the
compression of intercostal nerves, the patients encountered chest
pain (8).
Generally, pulmonary chondroma is one of the
clinical features of Carney's triad. Carney et al (9) first reported the disease in 1977. This
study included gastrointestinal stromal tumor, pulmonary chondroma,
extra-adrenal paraganglioma. If 2/3 clinical features are present,
it can be diagnosed as Carney's triad (9). Clinically, 2 features were often
observed in ~78% of patients (5). Generally, gastrointestinal stromal
tumors merged with pulmonary chondroma occurred more often in ~53%.
The syndrome is rare and predominantly affects young women;
however, the etiology remains unknown. Surgical resection was the
predominant treatment, which has a higher postoperative survival. A
total of 104 patients with Carney's triad were reported to have
survival rates of 10 and 40 years, for 100 and 73%, respectively
(10). For young women, a
gastrointestinal stromal tumor or pulmonary chondroma should be
considered as one of the clinical features of Carney's triad. In
addition, gastrointestinal stromal tumors and extra-adrenal
paraganglioma in Carney's triad were potentially fatal, and
eliminating the syndrome for patients with pulmonary chondroma was
necessary. The correct diagnosis for patients of Carney's triad is
essential to provide the appropriate treatment and result in a good
prognosis. To exclude Carney's triad, the 29 patients in the
present study underwent abdominal MRI examination and gastroscopy 3
months after surgery and no abnormalities were detected. As a
result of the heterochrony of the three tumors, lifelong follow-up
of patients with pulmonary chondroma is necessary.
A chest X-ray, enhanced CT scan of the chest or MRI
made it easier to identify the benign or malignant calcification
lumps. The CT is more commonly used, often displaying as round or
oval nodules, moderate soft tissue density, inhomogeneous density
with calcification and clear boundaries. Tumor diameter often
ranged between 1.0 and 4.0 cm with mild lobulation. No glitches,
satellite lesions or enlarged lymph nodes at the hilus of lung or
mediastinum were observed (11). In
the present study, patients with enhanced CT scan presented
similarly with those in the literature. Ultimately, pathological
diagnosis of the tumor is required.
Pulmonary chondroma can be easily misdiagnosed as
tuberculosis tumor, hamartoma (particularly cartilage hamartoma)
and peripheral lung cancer. In the present study, varying degrees
of calcification were observed in all cases. A total of 13 patients
were considered to have hamartoma, 14 patients were considered to
exhibit benign nodules, 2 patients with chest wall adhesion were
suspected of malignant infiltration and all patients were not
diagnosed as pulmonary chondroma.
Patients with a previous history of tuberculosis was
usually considered to have pulmonary tuberculoma (12). Tuberculoma was often located in the
dorsal segment of the lower lobe. Calcifications and cavity were
found in the lesion and were characterized by circular structures
by enhanced CT scan. The most common benign tumor of the lung was
hamartoma, which accounted for 5–10% of solitary pulmonary nodules
(13). Calcification looked like
popcorn or the image of fat density with enhanced CT scan and
allowed the differentiation of points of hamartoma. Notably,
calcification of pulmonary chondroma was smaller, mostly point-like
or scaly. In the present study, 13 patients (44.8%) were
misdiagnosed as having hamartoma. For older individuals who smoked
perennially, a cough with sputum and blood may easily be
misdiagnosed as peripheral lung cancer. Enhanced CT scans revealed
a burr-like structure of the tumor, pleural indentation and
inhomogeneous enhancement often accompanied by enlarged lymph nodes
at the hilus of the lung or mediastinum (14). Although the average age in the present
study was older, varying degrees of calcification were revealed in
the CT. Only 2 patients with chest wall adhesion were suspected of
malignant infiltration. In addition, previous medical history,
including primary malignant tumor and solitary pulmonary
metastases, was difficult to identify with non-calcified pulmonary
chondroma.
Although primary lung tumors are benign tumors of
cartilage, there remains the possibility of malignant
transformation. Mei et al (15) reported a case of giant primary
mesenchymal chondrosarcoma of the lung, followed-up for 6 months
and this patient succumbed to tumor recurrence and metastasis
(15). Certain patients succumb to
Carney's triad as a result of malignant alteration of lesions.
Therefore, the patients with Carney's triad must be given a medical
check periodically. It was more difficult to identify malignant
pulmonary chondroma with chondrosarcoma on the image presentations.
Surgical resection was the preferred treatment for pulmonary
chondroma. With the advantages of less trauma and faster recovery,
thoracoscopy or subaxillary minithoracotomy was used as the
preferred treatment (16). In the
present study, 23 cases were completely resected without residual
tumor or postoperative recurrence.
Pulmonary chondroma is a rare benign tumor of the
lung and the etiology remains unknown. It grows slowly with hidden
clinical symptoms, often identified by routine medical examination.
Pulmonary chondroma can be easily misdiagnosed as a tuberculosis
tumor, hamartoma (particularly cartilage hamartoma) and peripheral
lung cancer. As a result of the possibility of the malignant
transformation, complete surgical resection is the best treatment.
Pulmonary chondroma are possibly an initial clinical presentation
of Carney's triad; therefore, following the diagnosis of pulmonary
chondroma, further examination is required to exclude the Carney's
triad.
Acknowledgements
The authors would like to thank Dr Xiaoguang Guo of
the Department of Pathology, Nanchong Central Hospital, The Second
Clinical Institute of North Sichuan Medical College (Nanchong,
China) for the support and assistance.
References
|
1
|
Ishii H, Akiba T, Marushima H, Kanetsuna Y
and Morikawa T: A case of bilateral multiple pulmonary chondroma:
Necessity of follow-up for Carney's triad. Gen Thorac Cardiovasc
Surg. 60:534–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoekstra MO, Bertus PM, Nikkels PG and
Kimpen JL: Multiple pulmonary chondromata. A rare cause of neonatal
respiratory distress. Chest. 105:301–302. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bateson EM: Histogenesis of intrapulmonary
and endobronchial hamartomas and chondromas (cartilage-containing
tumours): A hypothesis. J Pathol. 101:77–83. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodriguez FJ, Aubry MC, Tazelaar HD,
Slezak J and Carney JA: Pulmonary chondroma: A tumor associated
with Carney triad and different from pulmonary hamartoma. Am J Surg
Pathol. 31:1844–1853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carney JA: Gastric stromal sarcoma,
pulmonary chondroma, and extra-adrenal paraganglioma (Carney
Triad): Natural history, adrenocortical component, and possible
familial occurrence. Mayo Clin Proc. 74:543–552. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ammar A, El Hammami S and Sellami Kamoun
N: Chondromas-a rare lung tumour: Rev Mal Respir. 22:826–827.
2005.(In French).
|
|
7
|
Silva VA, Kataguiri P, Trufelli DC, Matos
LL, Neves-Pereira JC and Campos JR: Pulmonary hamartoma as a
differential diagnosis of breast cancer metastasis: Case report. J
Bras Pneumol. 33:738–742. 2007.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allan JS: Rare solitary benign tumors of
the lung. Semin Thorac Cardiovasc Surg. 15:315–322. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carney JA, Sheps SG, Go VL and Gordon H:
The triad of gastric leiomyosarcoma, functioning extra-adrenal
paraganglioma and pulmonary chondroma. N Engl J Med. 296:1517–1518.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Smyrk TC, Young WF Jr, Stratakis
CA and Carney JA: Gastric stromal tumors in Carney triad are
different clinically, pathologically, and behaviorally from
sporadic gastric gastrointestinal stromal tumors: Findings in 104
cases. Am J Surg Pathol. 34:53–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strano S, Ouafi L, Baud M and Alifano M:
Primary chordoma of the lung. Ann Thorac Surg. 89:302–303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fain O: Pulmonary tuberculoma. Rev Prat.
55:17512005.(In French). PubMed/NCBI
|
|
13
|
Dragoumis DM, Boudalaki ES, Assimaki AS
and Tsiftsoglou AP: Pulmonary hamartoma masquerading lung
metastasis in a woman with inflammatory breast cancer. Breast J.
18:486–488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokota H, Shigeta A, Edo H and Niijima M:
Peripheral lung cancer effectively diagnosed with virtual
bronchoscopy. Nihon Naika Gakkai Zasshi. 96:781–783. 2007.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mei B, Lai YL, He GJ, Shou YN and Liu J:
Giant primary mesenchymal chondrosarcoma of the lung: Case report
and review of literature. Ann Thorac Cardiovasc Surg. 19:481–484.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ludwig C, Zeitoun M and Stoelben E:
Video-assisted thoracoscopic resection of pulmonary lesions. Eur J
Surg Oncol. 30:1118–1122. 2004. View Article : Google Scholar : PubMed/NCBI
|